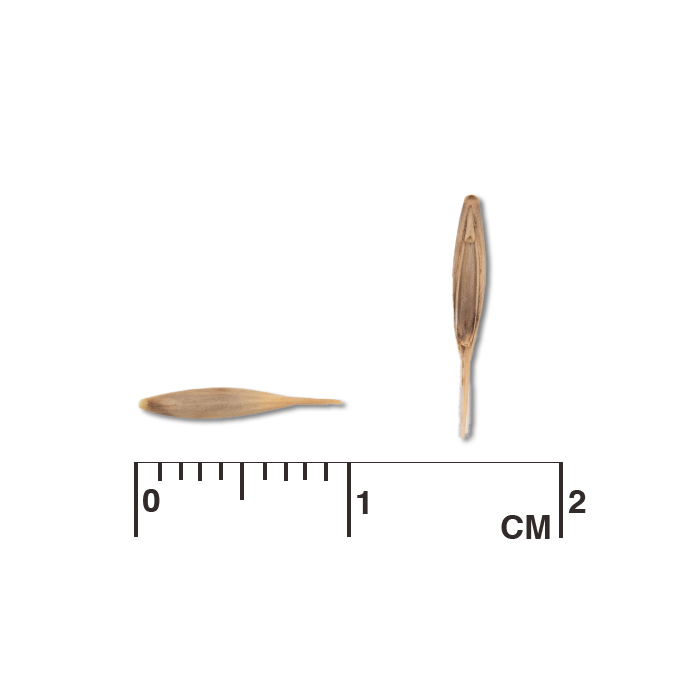
Native Seed Production Manual
Native Seed Production Manual parkecag
About the Manual
The intent of this manual, once completed, is to provide basic information for native seed production of nearly 100 species of the tallgrass prairie flora of the upper Midwest. The information presented is compiled from published accounts coupled with native seed production experience at the Tallgrass Prairie Center at the University of Northern Iowa. Critical to this effort were publications from the USDA-NRCS Plant Materials Program, published research articles and technical notes, personal communication with other native seed growers, and Internet resources. Botanical nomenclature follows Flora of North America, wherever possible. Common names follow USDA Plants Database.
The first edition of this manual was written in 2007 with support from USDA NRCS, the Tallgrass Prairie Center, University of Northern Iowa, and the Iowa Crop Improvement Association. A pdf of the original manual can be downloaded here.
The updated and expanded second edition is being produced as an eBook and made available for free on the Tallgrass Prairie Center website. For those who prefer a print copy, printable versions (pdf) of each general information chapter, species production guide, and appendix table will be provided. You are welcome to download and print the pdf versions to create a printed manual that includes the material of greatest importance to you.
This manual is a work in progress, and new information will be added as it is completed.
Funded by:
Iowa Living Roadway Trust Fund

University of Northern Iowa

Written and Edited by:
First edition (2007)
Greg A. Houseal, Program Manager, Iowa Ecotype Project
Second edition (2024)
Revised and expanded by Laura Fischer Walter, Plant Materials Program Manager
in collaboration with Green Iowa AmeriCorps members Bri Hull, Mallory Sage, Andrea Fager, and Ethan Dickey
and UNI students Laura Spies, Josie Hutchings, Alex Hanson, and Addison Owen
General Information
General Information parkecagThe first part of the manual consists of four chapters providing general information on greenhouse propagation, harvesting, seed processing, and issues of seed source and quality. Specific examples referencing native species are given to help illustrate the application of the techniques being described.
Propagation of Native Species
Propagation of Native Species parkecagRefer to Appendix A Table 1A [to be added soon] for establishment recommendations for individual species.
Propagation of native species through agronomic methods is necessary to scale up seed supplies for restoring native vegetation in highly fragmented landscapes such as the Midwestern Corn Belt of the US. However, information and techniques for propagating the diverse species needed for restoration are often in short supply, or are closely held secrets of private native seed producers. Here we present methods gleaned from a variety of sources, many of which we have used at the Tallgrass Prairie Center in production of source-identified stock seed for regional native seed growers.
This chapter focuses on establishment of production plots using transplants grown in a greenhouse. This approach has advantages: making efficient use of small amounts of stock seed and achieving more reliable seedling establishment (hence, efficient use of prepared plot space). However, it requires specialized facilities and involves considerable labor. Direct seeding is an effective method for establishing some native prairie species, notably the dominant grasses. Preparing a clean, weed-free seed bed and ensuring that seed is planted at the right depth (i.e., not buried too deeply) are crucial. In the species production guides, we provide estimates of the amount of seed needed, row spacing, and planting depth for direct seeding. However, we do not have experience with direct seeding all of the species and include a disclaimer to that effect when applicable.
Most of the species produced at the TPC have been grown in single-species plots of up to ½ acre or in 4-ft wide rows up to 150-ft long. Some growers produce seed using native or introduced grasses as cover crops within forb rows, in mixed cropping systems of a few species, or by reconstructing prairies for the purpose of seed harvest.
Wild species grown for seed production may show signs of adaptation to production conditions over several generations. At each step in propagation, there is potential for selection for genetic traits that improve fitness in an agronomic production system but may alter the ability of the seed to produce plants capable of establishing, surviving, and reproducing in the wild. Within each of the following sections, we will point out the risk of unintended selection and strategies for avoiding it. These strategies are also summarized in the Chapter: Seed Source and Quality. They will sometimes be in tension with the need for private seed companies to produce a profitable crop.
- Seed Dormancy
Dormancy describes seed that does not germinate even when presented with favorable conditions of moisture, temperature, and light. Dormancy is an adaptive trait, allowing germination to occur over time and in the proper season. This vital trait prevents the germination of all seeds at a time that might be suboptimal, or even lethal, for seedling establishment. Staggered germination over time is normal, even with stratification, and should be expected when propagating native prairie species.
The benefits of removing dormancy are twofold: First, more seeds germinate in a shorter period of time, which means limited and costly greenhouse space is used more efficiently. Second, increased germination means that more individual plants will potentially establish, flower, and reproduce, contributing their genetic diversity to the next generation.
There are two main categories of dormancy – primary and secondary. Primary dormancy occurs when seed is dormant upon dispersal, which is typical of many prairie species. Species with secondary dormancy produce seed with the ability to germinate readily upon dispersal (fresh); however, the seed may enter a dormant state if conditions aren’t favorable. Many woodland spring ephemeral species belong to this category of dormancy.
Within the two primary categories, there are several types of seed dormancy, and appropriate strategies are required to remove each type (Table 1). The most common type of dormancy in prairie plants of the grass and sunflower families is physiological dormancy, resulting from biochemical compounds that inhibit germination. The compounds may be produced in the seed itself or translocated to the seed from the plant prior to dispersal. Abscisic acid (ABA), for example, prevents premature germination of mature seeds in the seed head, before dispersal from the parent plant. Concentrations of germination inhibitors, like ABA, decline over time, allowing the seed to germinate. Physiological dormancy may respond to chemical treatments such as gibberellic acid or ethylene which counteract ABA. Another type, physical dormancy, is due to a physical characteristic of the seed; for example, the seed coat may be hard or waxy or otherwise impermeable to water and gas exchange, thus inhibiting germination. Species in the sumac, legume, geranium, and buckthorn families have these characteristics. Seeds with physical dormancy require scarification to remove these barriers. A third type is morphological dormancy: the embryo within the seed is underdeveloped upon dispersal, and warm, moist conditions are generally necessary for maturation (55 to 65 °F, 13 to 18 °C). Species with this type of dormancy are found in the parsley, buttercup, arum, lily, and iris families, among others (Baskin and Baskin 1998). Many seeds may have a combination of morphological and physiological dormancy types, i.e., morphophysiological dormancy, a subtype of which is sometimes referred to as double dormancy. Seeds of these species are often slow to germinate and may require lengthy stratification or repeated stratification at different temperatures. Examples of morphophysiological dormancy are found in the lily, gentian, buttercup, and parsley families. Combinational dormancy means that seeds have both a physical barrier to germination and biochemical inhibitors. Such seeds need both scarification and stratification in order to germinate. Some legumes and members of the borage and buckthorn families fit in this category.
Table 1. Types of dormancy associated with family groups and strategies for breaking dormancy (Adapted from Baskin and Baskin 1998).
Type of Dormancy
Cause
Removed by
Physiological
Biochemical inhibitors of germination (i.e., abscisic acid)
Cold and/or warm stratification, treatment with gibberellic acid (GA) or ethylene, light or dark conditions
Most common form of dormancy in the grass and sunflower families
Physical
Seed coat impermeable to water/gases
Scarification to simulate natural breakdown of barriers to water uptake
Found in legume, sumac, geranium, and buckthorn families
Morphological
Underdeveloped embryo
Conditions for embryo development, usually warm, moist conditions
Found in parsley, buttercup, arum, and lily families
Morphophysiological
Underdeveloped embryo and biochemical inhibitors
Lengthy stratification or a sequence of stratification periods at different temps; some species respond to GA
Found in gentian, buttercup, parsley, and lily families
Combinational
Physical barriers and biochemical inhibitors
Scarification (including wet heat) and stratification
Found in borage, buckthorn, and some members of legume family
Choosing Dormancy Breaking Treatments
When planning to propagate native species for seed production, it is important to consider the likelihood of seed dormancy and choose methods to effectively reduce it in that species. Within a plant species and population, individuals vary in their degree of dormancy due to complex genetic traits. If dormancy is not broken, individuals carrying this trait will be eliminated from the production population.
Several sources for finding dormancy breaking information are listed in the references for this chapter, including scientific publications, native plant nursery websites, and a searchable plant propagation database. Information in these sources comes from either scientific studies of germination or practical experience, or both. When different sources disagree significantly, it may be advantageous to divide the seed and subject different samples to each of the suggested treatments. It is also wise to consider the geographic source of the information, as levels of dormancy may differ within a species along a north-south gradient of its natural range (or altitude in other regions). Use caution in applying suggestions from native plant nursery websites: dormancy is a genetic trait that can be diminished in a production population over a few generations unless the grower has applied dormancy-breaking strategies.
For some species, little to no information is available. In those cases, first consider the natural timing of seed dispersal for the species. If its seed typically disperses in fall, cold stratification of different lengths is a good starting point. You may also find protocols for closely related species, divide the seed, and try a range of treatments based on what has worked for other members of the genus. Baskin and Baskin have published a more empirical method called the ‘move-along experiment’ that helps to determine the set of temperature treatments needed to germinate one of these unknowns. If you manage to ‘crack the code’ on a difficult species, consider sharing your techniques on the RNGR Propagation Protocols Database.
- Seed Treatments
Scarification
Scarification is a technique that simulates the natural disintegration of the seed coat to initiate germination. A hard or waxy coat will not allow the seed to soak up (imbibe) the water needed for germination until the seed coat breaks down. Seed is scarified either through natural processes such as weathering, abrasion, or partial digestion, or through artificial techniques. Seeds have natural openings for water uptake and these weather or wear away first, especially in seeds with hard seed coats, allowing the seed to imbibe water so germination can occur. The trick of scarification, then, is to accelerate the process of weathering these natural openings so seeds can imbibe water, but stopping short of damaging the seed. Some simple scarification techniques are presented here.
Sandpaper wood blocks – These blocks can be constructed using rubber cement to glue a sheet of fine-grain sandpaper to each of two flat plywood boards. Lay one sand block on a tray and use light pressure and a circular motion to move the other sand block on top of a quantity of seed sandwiched in between the two blocks. Adequate scarification is achieved after a minute or two, when seed begins to look dull.
Percussion scarification – Seeds are shaken vigorously inside a heavy glass bottle for a few minutes. Allow ample room for all of the seeds to impact the sides of the bottle. This technique is considered less aggressive and less likely to damage seeds than the sand block method. In a variation of percussion scarification, a pneumatic paint shaker was modified by Khadduri and Harrington (2002) to scarify the very hard seeds of native locust tree species (Robinia neomexicana, R. pseudoacacia).
Wet heat – Pour boiling water (212°F, 100°C) over the seeds just enough to cover them and allow to cool to room temperature, or immerse seeds in boiling water for five to twenty seconds and remove to rinse and cool. This technique is reserved for certain species and is not broadly recommended. Some species will also require stratification after wet heat. This is effective for New Jersey tea (Ceanothus americanus), hairy puccoon (Lithospermum caroliniense, followed by 90-day cold stratification), and reportedly for false gromwell (Onosmodium molle).

Image: Hairy puccoon, Lithospermum caroliniense, nutlets subjected to boiling water scarification prior to being cold/moist stratified for 90 days. Commercial scarifiers are also available from seed equipment manufacturers, such as a Forsberg scarifier, a sandpaper-lined cylinder with metal paddles that turn and agitate the seed. This is a very aggressive method and only a few seconds are generally needed. Precious seed can be reduced to flour if left on too long. Seed may already have been scarified as part of the cleaning process (e.g., if a scarifier/brush machine was used for dehulling legume seeds). Contact your seed vendor/producer to determine if the seed has been cleaned with a scarifier/brush type machine.

Image: A Forsberg scarifier, to be used with caution. Stratification
Stratification is a process whereby seeds are placed in a moist medium such as clean sand or vermiculite at appropriate temperatures for a specified period of time. The idea is to mimic critical conditions necessary for germination that seeds are exposed to naturally in the environment after dispersal. Generally, if seeds are dispersed in autumn, they may require cold, moist stratification. If dispersed in late spring or early summer, they may require warm, moist conditions, or warm followed by cold stratification, as in Michigan lily (Lilium michiganense). Mix seed with an equal amount of moist, sterile sand, sawdust, peat, or vermiculite and place in a zip-closure bag or small plastic container. Avoid excessive moisture; water should not be pooled anywhere in the bag or container. Use vermiculite if working with species adapted to drier conditions to minimize the risk of rot. If it is desirable to separate the seed from the medium after stratification, one strategy is to place the seed in a small fine-mesh (organza) bag such as those used for wedding favors, then sandwich the bag between layers of moist stratification media.

Image: Seeds of common boneset (Eupatorium perfoliatum) in containers of moist silica sand placed at 40°F for 60 day stratification. Effective temperatures for cold stratification are from 32 to 45 °F (0 to 10 °C), with 41 °F (5 °C) considered optimal for many species (Baskin and Baskin 1998). Some species require as little as ten days, others as long as 120 days. A few species, among them American vetch (Vicia americana) and butterfly milkweed (Asclepias tuberosa), will germinate at these temperatures, so check bags weekly to look for emergence of the radicle and plant immediately if this occurs. Some species may germinate best when stratified under natural winter temperature fluctuations (for example, in an unheated building). If sowing seeds in flats for outdoor stratification, cover with screen mesh to protect seeds from being displaced by animals or heavy rains. Cold frames can be used for stratification and extending the growing season in the spring. Effective temperatures for warm stratification are from 68 to 94°F (20 to 35°C), with 68 to 76°F (20 to 24°C) optimal for many species with this requirement (Baskin and Baskin 1998).
Rhizobium Inoculation
Rhizobium are types of nitrogen-fixing bacteria that live symbiotically with the roots of many species, typically forming nodules on rootlets of the plant. They “fix” nitrogen by converting gaseous nitrogen from the air spaces in the soil into plant-available ammonia nitrogen, which directly benefits the host plant. The plant, in turn, provides carbohydrates for the rhizobium. Strains of rhizobium have been isolated and are commercially available for groups of species, notably the genera Amorpha, Lespedeza, and Dalea. Rhizobium comes as a black powder that is mixed with the seed just prior to planting. Greenhouse-grown seedlings of legumes benefit from rhizobium inoculation. It may be unnecessary to inoculate with rhizobium, however, if seedlings will be planted within a few weeks after germination into native soil where rhizobium naturally occurs.
Mycorrhizal Inoculation
Mycorrhiza means “fungus root” and describes a symbiotic relationship between plants and fungi. This is common in many, if not most, plant species. Mycorrhizal fungi are naturally occurring in healthy soil, but may need to be provided for soils that have been fallow, flooded, or eroded over long periods. Sites disturbed by extensive and long-term construction grading or altered by mining will also benefit from inoculation. Commercial inoculum consisting of endomycorrhizal spores, host plant roots, and a sterilized medium is now available, and this can be incorporated into the soil at the time of seeding or transplanting. Inoculation of the site with healthy soil from a different location is another option.
- Greenhouse Propagation
Once viable seed is obtained and pre-treated to remove dormancy, it is ready to plant in the greenhouse. Critical factors include a suitable container and potting medium, water, soil temperature, light, and air.
Containers
Containers should provide good drainage and space for root development and yet be small enough to provide efficient use of potting medium and bench space. Nurseries interested in retail seedling/plant production will first germinate seeds in flats, which take up much less greenhouse space. Seedlings are then transplanted into disposable trays with perforated pull-apart planting cells for retail marketing.
Seedlings for transplant into seed production rows can be grown in plug trays or a modular reusable “cone-tainer” and tray system. In recent years, the TPC has had success with 2.5” deep, 73-cell plug flats that are ridged to direct root development downward and have ¾” bottom openings to encourage root pruning and the formation of firmly rooted plugs for transplanting. It is easier to maintain even watering with these plugs than deeper cone-tainers, and a transplantable plug can be produced in a shorter time for most species. In addition, the shallower depth of holes required for transplanting into production rows means that the holes are less apt to collapse and require re-digging. We observe little mortality of these plugs transplanted into prepared fields in spring, generally in May to early June. In very dry years, we provide supplemental water once or twice a week for the first few weeks in the field, but normal rains are usually sufficient for establishment of transplanted plugs.



Images (left to right): seedlings of common boneset (Eupatorium perfoliatum) with first true leaves in a germination tray (with wooden chopstick for scale); one of the seedlings extracted with tweezers to show roots at suitable stage for dibbling into plugs (the chopstick serves to dibble a planting hole in each plug); seedlings in plugs two weeks after dibbling.
In the past, the TPC used Stuewe “cone-tainers” for greenhouse propagation. Cone-tainers are designed for accommodating taproot growth in conifer tree seedlings. They worked well for perennial prairie species – particularly those that put down tap roots like compassplant (Silphium laciniatum), butterfly milkweed (Asclepias tuberosa), and Baptisia spp. Various sizes of cone-tainers are available in yellow UV-stabilized plastic for longer life. The so-called “fir” cells, approximately 6.5 in deep with a 1-in. diameter at the top, work well for most native species. Each tray accommodates 200 cone-tainers, 100 cone-tainers per square foot, so it’s also an efficient use of limited bench space. Planting “dibbles” are available that match the size and shape of the cone-tainers. The cone-tainers and dibble system allow the roots of seedlings to be planted deeply enough so that roots can tap into subsoil moisture. Irrigation isn’t necessary, especially with spring or fall transplanting when rains are more frequent.
There is no single “best” container for prairie plant propagation. Some species that form fleshy basal rosettes in the first year of growth (e.g., Lobelia spp.) seem to benefit from being started in wider, shallower plugs or pots. For species that are sensitive to root disturbance during transplanting, consider producing plugs in biodegradable containers such as coir pellets or Stuewe Zipset Plant Bands, made from milk carton paperboard.



Images (left to right): cardinalflower seedlings (Lobelia cardinalis) nearly one month after dibbling from a germination flat into 6-pack trays; the same seedlings with leafy basal rosettes three weeks later; and plants ready for transplanting to the field six weeks after dibbling into 6-packs.
Potting Medium
A good potting medium should be light enough to allow for good root development, provide adequate drainage, and have enough fertility for seedlings to grow quickly in the greenhouse environment and become large enough for transplanting in a reasonable amount of time. It should also be sterile, meaning free of weed seed and disease organisms. We recommend a soilless mix (less than 20% soil) consisting of 10% sterile soil and 10% composted manure, about 50% milled peat moss or coco coir, and the remainder equal parts perlite and vermiculite.
Caution: All of these materials are extremely dusty. Wear a high-quality particulate mask when handling and mixing materials. Gloves and dust goggles are also recommended. Materials should be moistened before mixing to reduce dust.
Pre-mixed sterile potting mix for plug production can be purchased in bulk from nursery and greenhouse supply companies. Some formulations include potentially beneficial additives such as mycorrhizal inoculum (generally a single species) and biological fungicide (a bacterial species). For propagation of most prairie plants, we supplement “general purpose” mixes by adding additional perlite (2.8 gallons), vermiculite (2.8 gallons), and coated, slow-release fertilizer pellets (19 oz, 15-9-12 formulation) per 3.8 cubic foot compressed bale (7 cu ft expanded). Seedlings are susceptible to damage from over-fertilization, particularly legumes. Controlled-release fertilizer has the added benefit of continuing to provide fertility after transplantation of seedlings into production plots. A drill attachment designed for mixing drywall joint compound helps combine these ingredients while also breaking up chunks of compressed media.

Image: Small amounts of prepared soilless mixes can be amended and mixed efficiently with a joint compound mixer. Goggles and a particulate mask provide protection from dust particles. If you prefer to mix your own potting medium, the recipe below makes approximately 1 cubic yard of soilless mix (enough for 40 trays of 200-cell “fir” cone-tainers). To improve the sustainability of this mix, you could substitute coco coir for the peat moss. Coir is available in compressed, dry bricks that expand greatly when moistened (one 11-lb brick expands to 3 cu ft). If mixing on the floor, sweep and vacuum the area before mixing to remove seeds, debris, and other contaminants:
Peat moss (4 cu. ft/bag) or coir (see above text) 2 bags (8 cu. ft) Vermiculite (medium texture, 4 cu. ft/bag) 1/2 bag (2 cu. ft) Perlite (4 cu. ft/bag) 1/2 bag (2 cu. ft) Sterile soil two 5-gal buckets Composted (sterile) manure manure 40-lb bag Osmocote® Plus fertilizer 15-9-12 (180 days) 8 lb Screen peat moss, soil, and composted cow manure through a ½-in. mesh hardware cloth screen to break up or remove large pieces that would tend to clog and create air pockets as cone-tainers are being filled. Add remaining ingredients, mix with shovels on a clean floor, and fill trays.
Strategy for Filling and Seeding Cone-tainers or Plug Trays
We fill several plug trays at a time within a larger, heavy duty garden tray, banging the large tray several times to settle the medium and sprinkling the plugs with water after each addition of soilless mix, until the level of the medium is consistent across the plugs and flush with the top of the plug tray. It will continue to settle somewhat as the trays are watered in, leaving a little space at the top for water to puddle.

Filling plug flats with potting mix. When filling trays of cone-tainers, tamp the tray on the floor to firm potting medium and remove large air pockets. Avoid overfilling. Leave about ¾-in. unfilled space at the top of each cone-tainer. This space acts as a reservoir during watering, allowing the water to seep in slowly, helping to saturate the entire soil column. Water cone-tainers frequently for a day or two before planting to fully hydrate the potting medium. Refill any cone-tainers that may have settled excessively.
Attempt to sow several seeds per cone-tainer or plug cell. If seed has been mixed with damp sand or other medium for stratification, it may be impossible to distinguish small seeds from the medium. If this is the case, place the damp seed/medium mixture into a shallow dish and mix thoroughly to evenly distribute the seed within the medium. Use a small, flat implement (point of a knife or wood popsicle stick) to place a small amount of mixture in each cone-tainer.
Getting an appropriate number of seeds per cell is guesswork with tiny seeds, but experience will improve efficiency. Thinning may be necessary if too many seeds germinate in each cell, and this could lead to unintended selection. Blank cells will result if no viable seeds were planted, wasting greenhouse space and making it more difficult to achieve even watering. For larger seeds with high purity and viability (90% or more), one to three seeds per container is adequate. Increase accordingly for seed of poor or unknown quality. Cover with no more than 1/8- to ¼-in. of soil for most species. Caution: Very tiny seeds should not be covered! Additional information and precautions on sowing seed of specific species can be found under the following sections on light, temperature, and water requirements for propagation.


Image: Samples of stratified seed are spread evenly on the surface of prepared germination trays. Small seeds such as these skyblue aster achenes (Symphyotrichum oolentangiense) are very lightly covered with soil.
One strategy to ensure even planting of plug trays is to start seed in germination flats, then prick out seedlings with forceps (tweezers) and transplant them individually into plugs when ready, generally when the first set of true leaves has emerged. There is a risk that individuals with genetic traits that cause them to germinate later could be selected out of the population by this practice. To avoid selection against later germination, retain the germination flats and add later emerging seedlings to plugs when they are ready.

Image: Seedlings from germination trays are dibbled into plugs using forceps, ensuring that all plugs in a flat are filled. This practice is labor-intensive but makes efficient use of seed and greenhouse space. Light
See Appendix A, Table 2A for recommended planting depth and light requirements
Natural light should be sufficient for seedling establishment in the greenhouse from mid-March through mid-September. Sow seeds in early February and expect germination and emergence to occur over a two- to six-week period. Greenhouse-grown seedlings grow well with only natural light through March and April and into May, when transplanting into production plots begins. Some species require light for germination. These are typically small-seeded species, including Culver’s root (Veronicastrum virginicum), mountainmints (Pycnanthemum spp.), grass-leaved goldenrod (Euthamia graminifolia), bonesets and Joe Pye weeds (Eupatorium and Eutrochium spp.), great blue lobelia (Lobelia siphilitica), and prairie sage (Artemisia ludoviciana). These do best if sprinkled on top of the soil surface and kept continually moist until the seed leaves (cotyledons) are evident.
Temperature
Germination will occur throughout a range of temperatures but will be slower with less than optimal temperatures. The risk of fungal pathogens and rot increases if seed does not germinate and is non-dormant. Warm-season grasses and legumes germinate best in warm soils greater than 70°F (21°C ). Cool-season grasses and many forb species will germinate more readily in cool soil temperatures of 40 to 50°F (5 to 10°C) and may cease germination at temperatures above 77°F (25°C). Soil temperature in the planting trays fluctuates with greenhouse air temperature (72°F, 22.2°C daytime and 60°F, 15.5°C nighttime). Pulses of emergence occur on sunny days with some species, presumably because an optimum soil temperature has been reached from solar heating. Covering plug trays or cone-tainers with translucent plastic increases soil temperature and improves the germination of species that require warm soil temperatures. Use this technique with caution. Lethal temperatures can occur quickly under the plastic with full summer sun. Plastic should be removed as soon as the first seedlings emerge to avoid overheating new seedlings. Cooler soil temperatures can be achieved by setting trays on the floor. If sowing seed in germination flats, precise regulation of soil temperature can be achieved with propagation mats. Propagation mats are placed under the flats and are plugged into an electrical temperature control box. Soil temperature in the flats is regulated by a soil temperature probe from the control box inserted into the potting soil of one of the flats. These are commercially available at reasonable cost from nursery or greenhouse supply companies.
Watering
Proper watering is critical to greenhouse propagation. Watering methods must be adjusted depending on the growth stage of the plants. It is important to keep the soil surface moist until germination has occurred, especially for small seeds that require light and are sown directly on the soil surface. An automated mist system is helpful during this stage of propagation. If using a wand or watering can, use a sprinkler head that produces small gentle droplets and low pressure so that watering doesn’t dislodge seed, force it deeper into the soil, or splatter it out of the containers.

Image: A gentle shower from a watering wand soaks young seedlings without flooding or dislodging them. Established plants should be watered thoroughly about once a day, until water drains from the plugs, ensuring that the entire soil column is moistened. Allow the soil to drain and the surface soil to begin to dry slightly between waterings. Shallow, light watering will cause the lower portion of the soil to dry out and root growth to stall. Containers that are overfilled with soil are prone to underwatering since the water can’t pool on the surface and gradually soak in. Overwatering saturates the soil, depriving the root zone of air and creating conditions conducive to decay. Containers and potting medium that allow for proper drainage will help prevent over-watering. Cool, cloudy days reduce watering needs. More frequent watering (two to three times daily) is required on hot, sunny days and for larger plants with fibrous roots that fully occupy the container.
Excessive watering can also damage shoots. Healthy-looking plants will suddenly fall over, appearing to be cut off at the soil level. This is known as “damping off” and is caused by a fungus. Legumes are particularly susceptible to this condition, but it can affect other species as well, especially if they are planted too densely. Sprinkling a layer of fine chick grit (found in the feed section of farm supply stores) over the top of the soil surface after seeding or transplanting will help dry the soil surface and wick water away from the stems. Maintaining good air circulation will evaporate excess water from stems and the soil surface and minimize the risk of damping off. Place additional fans near benches with species known to be susceptible. Thinning may be necessary to improve air circulation. Watering pots/containers from below by setting them in a pan of water until the soil wicks up moisture may also help. Washing and sterilizing containers, benches, and equipment and using sterile potting media also help reduce the risk of damping off.


Image: A topping of chick grit keeps the surface around seedlings dry, helping to prevent outbreaks of damping off fungi.
- Transplanting Seedlings
Seedling Development/Timing
The key to successful transplanting of native perennial species is strong root development. Ideally roots should fully occupy the entire soil column so that when the plant is removed from the container, the soil and roots remain intact as a “plug” (i.e., they retain the shape of the container). Grasses and forbs with fibrous roots form beautiful plugs after a few weeks of growth and present little challenge in transplanting. Species with taproots (e.g., Baptisia spp., compassplant, Desmodium spp., butterfly milkweed) develop a thickened fleshy taproot within a few weeks after germination in a greenhouse, but the taproot itself may not be enough to hold the plug intact when transplanting. However, if growth is allowed to continue until the taproot reaches the opening at the bottom of the container, the taproot will air-prune, and fine lateral root growth will be stimulated. These fine lateral roots will help considerably in holding the plug intact when the seedling is removed from the container for transplanting. Slower growing forbs and shrubs require more time for roots to develop adequately for transplant. This process is accelerated by using the 2.5” deep plug trays with ridges and large bottom openings, as opposed to cone-tainers.

Image: Plugs of a taprooted species, longbract wild indigo (Baptisia bracteata, cream wild indigo). Firmly rooted plugs were produced by growing seedlings in air-pruning containers with grooves and large bottom openings. Seedlings are prone to top-kill when transplanted directly from the greenhouse into the field. Greenhouse-grown seedlings are pampered: they have been protected from drying wind, harsh sun, and herbivores. Robust greenhouse-grown seedlings of many native species can tolerate the stress and will regrow quickly if they have strong root development and adequate soil moisture. A better approach, however, is to acclimate seedlings gradually to outdoor conditions with a process called “hardening off.” A week or two before transplanting, set flats or trays outside for a few hours each day from mid-morning to mid-afternoon in a place sheltered from strong winds and full sun. The idea is to acclimate the plants gradually to outdoor conditions of wind and sun. Strong winds and heavy rains should be avoided. Another option is to move flats/trays into a cold frame (unheated greenhouse) and roll up the sides or open the side vents to allow natural airflow and some direct sun to the plants.
The ideal time for transplanting is in the spring just after the last frost-free date for your region. Rains are more reliable at this time of year, the sun less intense, and plants have the entire growing season to establish and flourish. If transplanting during the summer months, check for adequate soil moisture. Be prepared to water regularly and deeply until plants are established. Transplanting in the fall (early to mid-September) may be another option. Plants transplanted after mid-September may not have enough subsequent root growth to anchor them in the soil, making them vulnerable to frost-heaving.
Bare Soil vs. Weed Barrier
To establish seed production plots, seedlings can be transplanted into weed-free bare soil or a weed barrier. Transplanting into killed sod works well for natural landscaping with a mixed assemblage of prairie species but is not recommended for seed production plots. The dead sod interferes with cultivation and hoeing should weeds become a problem.
Aggressive clonal species like wild bergamot (Monarda fistulosa), prairie coreopsis (Coreopsis palmata), Culver’s root (Veronicastrum virginicum), and prairie cordgrass (Spartina pectinata) can be successfully established as seed production plots in bare soil. Good weed suppression, as always, is critical. Pre-emergent herbicide (Pendulum®, Prowl®) can be used on the site after transplanting to inhibit weed seed germination. Be sure to water in seedlings after transplanting to settle soil around the root zone before applying pre-emergent. Otherwise rain may concentrate run-off of herbicide into freshly dibbled holes containing the new transplants, damaging roots or killing the seedlings. Do a small test plot on species not listed as tolerant on the product label. Read, understand, and follow all precautions and directions on the product label. Planting 8 to 12 in. apart in well-spaced rows (32 to 36 in.) will permit cultivation later that season for weed control. Do not cultivate as long as pre-emergent herbicide remains effective in suppressing weed seed germination. These aggressive clonal species will spread and create solid rows in two to three years. Seed production begins to decline after the third year and may drop off sharply after the fourth year as plants become root-bound within the rows. Aerating the soil with a turf-type aerating implement in the fall or early spring may extend plot productivity.
Weed barrier comes in a variety of materials: plastic film, woven fabric, paper, and fiber mats. Plastic film is inexpensive but less durable than most other types of barriers. Plastic creates abnormally moist conditions that can increase disease problems in some species but may be suitable for short-term (one to two-year) applications. Biodegradable plastics are available which begin breaking down after the first growing season. Plastic film can be applied mechanically using an implement that creates a flat or raised bed while simultaneously rolling out and securing the plastic. Irrigation tubing can be laid at the same time for species needing additional moisture. Plugs can be transplanted into holes dibbled through the plastic film. When using 4-ft wide plastic, we typically plant four plugs per row in rows about a foot apart. We adjust this plant spacing to accommodate larger species such as compassplant.


Image: Metal dibbles punch planting holes directly through plastic weed barrier. Most species are planted in rows of four spaced one foot apart.
As the name implies, a weed barrier suppresses weed growth by blocking light, physically obstructing shoot growth, and by solar heat sterilization of weed seeds in the soil. Weed barriers also conserve soil moisture, and plants grow more vigorously with a weed barrier than in bare soil. For these reasons, weed barriers aid establishment in the first year and increase seed production in the second year for most species. By the third year, aggressive clonal species will send rhizomes in all directions that the barrier would smother. For these species, plant in bare soil as described above, or use an inexpensive barrier that biodegrades or can be pulled up after the second season.
In the past, we used a durable, water- and gas-permeable weed barrier (DeWitt Pro woven landscape fabric) for long-term applications (up to ten years). This can be purchased in rolls 6’ x 250’ or 12’ x 300’ rolls. This type of weed barrier provides long-term benefits of weed suppression and moisture retention for less-competitive species that establish slowly. It’s also a good choice for species with taproots that remain where they are planted (non-clonal). However, removal and disposal of fabric weed barriers after many years in the field is very difficult, as perennial grasses and other weeds grow through the degrading barrier, uniting it with the soil.
Transplant seedlings of non-clonal species into weed barrier fabric 8 to 12 in. apart in blocks or rows to optimize the use of this costly product. Slits are cut in the fabric weed barrier using a tool made of modified sickle bar blades mounted on a wooden 2x4 with an attached handle. This device cuts four “x-shaped” 2-in. slits, 8 in. apart at one time. Cutting larger slits would allow more weeds to germinate and come up through the openings. Precise holes can also be burned into weed barrier fabric using a propane-fired hole cutter available from greenhouse and nursery supply companies.
Regardless of the type of barrier used, some hand weeding will be necessary, especially during the first growing season. Hand-pull weeds that emerge through the barrier openings while they are small, taking great care not to disturb new transplants. Pressing gently downward on the top of the newly transplanted plug while pulling adjacent weeds will protect it from being uprooted. A garden or dandelion knife can be used carefully to slice through the taproots of larger weeds. Slice into a taproot just below the soil surface, again taking care not to damage the roots of transplants. Hand weeding periodically throughout the first growing season will be required to give transplants the best chance of establishing the first year and producing a good seed crop in subsequent years.
How to Transplant Seedlings
Soil should be firm in all cases. If dibbling into bare soil, the soil should be rolled or packed to prevent dibbled holes from collapsing. Likewise, very dry soils resist dibbling. It may be necessary to sprinkle the area the day before or wait until a day or two after a soaking rain. Just before transplanting, liberally water seedlings to fully saturate the root plugs. This will make it much easier to remove the plugs from the trays or cone-tainers and provide extra moisture to the root zone after transplanting. To remove plants from a plug tray, use a stick or gloved finger to push up through the opening at the base of each plug. Hold the plant at its base and pull gently. If the plant does not release easily, try “massaging” to loosen the root plug from the sides of the cell. If the plants are in cone-tainers, hold a cone-tainer upside-down firmly in one hand, and rap the rim of the cone-tainer sharply on the palm of the other hand, using a flick of the wrist. The plug should slide out easily; repeat if necessary. (If plugs do not hold their shape upon removal, either the roots are not adequately developed or too much force is being used. Transplant success drops significantly if this happens!) Slide the intact plug into a dibbled hole – it should just fit, with the top of the root plug just at or slightly below the soil level. Pinch soil firmly around the top of the plug to seal in moisture, taking care not to bury the base of the shoot. The lighter soil-less mix can wick moisture away from the roots if left exposed. Be sure dibbled holes are deep enough to comfortably receive the full depth of the plug. Plugs forced into a too-small or shallow hole will often pop out of the ground after a good rain, exposing the root collar.


Image (left to right): Well-rooted plugs pop out of trays intact; plugs are "pinched in" to achieve good contact between roots and soil and topped with a thin layer of field soil to prevent moisture from wicking out of the potting mix.
- References
Baskin, C. C., & Baskin, J. M. (2003). When breaking seed dormancy is a problem: Try a move-along experiment. Native Plants Journal, 4(1), 17–21. https://doi.org/10.3368/npj.4.1.17
Baskin, Carol C., & Baskin, J. M. (2014). Seeds: Ecology, biogeography, and evolution of dormancy and germination. Elsevier / Academic Press.
Deno, N. C. (1993). Seed germination: Theory and practice. available for free download from the USDA National Agricultural Library at https://search.nal.usda.gov/permalink/01NAL_INST/27vehl/alma9916347016207426
Khadduri, N. Y., & Harrington, J. T. (2002). Shaken, not stirred - a percussion scarification technique. Native Plants Journal, 3(1), 65–66. https://doi.org/10.3368/npj.3.1.65
Native Plant Network, USDA Forest Service. (n.d.). Propagation protocols database. Reforestation, Nurseries, & Genetic Resources (RNGR). https://npn.rngr.net/propagation/protocols
Society for Ecological Restoration and Royal Botanic Gardens Kew. (n.d.). Seed information database. https://ser-sid.org/about
Harvesting Native Seed
Harvesting Native Seed sagemProper timing of seed harvest and use of effective harvest techniques increases the likelihood of producing a profitable yield of viable seed that will be effective for restoration of native vegetation. Harvest is clearly a critical step in seed production. It is also one of the steps that is most susceptible to unintended selection leading to adaptation of wild species to production systems. Wild populations have variation in inherited traits related to seed harvest such as the timing of maturation and the degree to which seed shatters or is retained on the plant at maturity. Variation in these traits provides wild populations with the ability to adapt to changing conditions and to disperse and establish without human intervention. Loss of these traits within production populations is possible when all seed in a field is harvested at peak maturity (eliminating the genetics of earlier and later maturing individuals from the harvest) or when most of the harvested seed comes from individuals that retain seed longer (i.e. are less prone to shattering). The risk of selection is compounded as harvested seed is used in expanding fields and/or planting new fields over several generations. Keeping the risk of selection in mind and utilizing strategies to avoid it is key to ensuring that commercially produced seeds retain genetic diversity and the ability to grow, survive, and reproduce as wild plants in restoration sites.
- Gauging Seed Maturity
Factors Affecting Seed Maturity and Dispersal
Gauging seed maturity is key to harvesting quality seed. Immature seed stores poorly, losing viability more quickly than mature seed. Determining the ideal time to harvest can be difficult. Within a population of native plants, there is genetic variation among individuals in the timing of seed ripening, and repeated harvests may be called for as seeds mature on different plants. In addition, seeds in different parts of an individual seedhead or inflorescence are often at different stages of ripeness. Seed maturity usually progresses from top to bottom of the seedhead in grasses and many prairie forbs. In forb species with flattened flower clusters, seed often matures first at the center of the inflorescence. Mature seeds may be quickly dispersed by gravity, wind, water, or animals, so it’s important not to delay harvesting. Some species forcefully eject seed at maturity (e.g., spiderworts, phlox, wild petunia, and violets) and require special harvesting strategies.

Conceptual diagram illustrating the optimal harvest time for capturing maximum mature seed yield, assuming the field is harvested mechanically on a single day. As seed ripens, seed shattering follows, and the rate of both processes increases over time. Timing the harvest carefully maximizes seed yield and quality. Environmental factors also influence seed maturation. Cold, moist conditions tend to delay seed maturity while hot, dry conditions may hasten it. Latitude also affects ripening since many plants flower and set seed in response to photoperiod. Flowering and seed set may be delayed if plants are grown northward from their origin, or hastened if moved southward. If moved a great enough distance north or south of their origin (greater than 300 miles) they may fail to set mature seed.

Three fields of butterfly milkweed (Asclepias tuberosus) showing differences in flowering phenology, photographed on July 3, 2023 at the Tallgrass Prairie Center, UNI. The northern Iowa zone (left) is nearly finished flowering, central Iowa zone (center) is just past peak flowering, and southern Iowa zone (right) is approaching peak flowering. Gauging Seed Ripening in Grasses
Seed ripening and timing of harvest varies by species, source of parent material, and environmental conditions. For example, cool-season grasses begin growth early in the growing season and consequently ripen earlier as compared to warm-season grasses. In grasses, there are roughly four stages of seed maturity: milk, soft dough, medium dough, and hard dough. Firm thumbnail pressure on the caryopsis, or grain, will help determine maturity. Grasses should be harvested at the hard dough stage, when firm thumbnail pressure slightly dents the caryopsis. Many grasses ripen about a month after flowering, and some do not hold seed long after maturity. Test ripeness by firmly striking the seed head against your palm; if some shattering occurs, the seed is ready to harvest. If it shatters with only gentle striking, harvest immediately. In some species, such as sideoats grama, visual assessment of the stand provides an indicator of maturity: the stand is ready to harvest when about 10% of the plants have started to shed seed.
Gauging Seed Maturity in Forbs
Generally, the seedhead itself or the stalk immediately below the seed head will begin to appear dry or discolored as the seed ripens. The leaves may also change color as the plant approaches dormancy. If the seed is easy to strip off or shatters out when the seed head is gently struck against the palm, seed ripening has begun. Some species have seed capsules that dry and split open when ripe, allowing seed to be released by wind, passing animals, or gravity. The spiderworts (Tradescantia), members of the day-flower family (Commelinaceae), are a special case: they drop seed from individual seed capsules as they ripen even while the bracts remain green and other flowers in the same cluster are in bud or blooming. Species with dispersal apparatus such as awns or hairs will appear dry and fluffy at maturity. It can be tricky to time the harvest of fluffy-seeded species that release their seed soon after maturity (many species in the aster family). Picking a few heads and examining the developing “seeds” (technically a type of fruit called cypselae or achenes) can help gauge approaching maturity. Seed that is nearly ripe will develop the mature color and separate easily from the receptacle.

A hairy mountainmint plant (Pycnanthemum pilosum) showing signs that it is ready to harvest: leaves are changing color and dropping and stalks beneath the seedheads are dry and brown. 
Three species of forbs showing capsules that have opened and begun releasing seed; they should be harvested immediately to avoid losing the crop. Left to right: bottle gentian (Gentiana andrewsii), swamp lousewort (Pedicularis lanceolata), and cardinalflower (Lobelia cardinalis). 
Fruit and seed ripeness varies within a seed production plot. Left: a field of butterfly milkweed with "pods" (follicles) at varying stages of ripeness. Center: Once pods split naturally, the seed rapidly blows away. Right: A pod that bursts in response to gentle pressure on the seam reveals chocolate-brown, mature seeds; this is the ideal time to harvest. 
Maturing seedheads on a sky blue aster plant (Symphyotrichum oolentangiense). The greenish receptacle near the center shows three indicators of approaching seed maturity in many members of the aster family: fluffy pappus, mature color of achenes, and easy separation from the receptacle. - Hand Harvesting Techniques
Hand harvesting is time- and labor-intensive and not practical for most large projects, but it is an important way to collect the seed of native species that are commercially unavailable and/or impractical to harvest mechanically. Such species may be low or high growing species, early or late ripening species, species with explosive seed capsules, or uncommon or patchy species in native prairie. There are also situations in a seed production system when hand harvesting may be needed, for instance, when collecting seed from early maturing individuals to ensure conservation of genetic diversity. Efficiency can be improved by keeping both hands free to harvest by simply fastening collection bags and containers around the waist with a bungee cord. Hand harvesting aprons are also available from some native seed businesses and garden suppliers. A plastic comb can be used to strip seed from grass stems. Scissors, clippers, or a hand sickle are useful for cutting tough stalks beneath seed heads.

Three situations in which hand harvesting was warranted: Left - stripping seed from sedge plants in a plot that was partially invaded by another sedge species (note hands free technique); Center - using a sickle to cut early maturing individuals in a prairie Junegrass plot (the plot was later combined); Right - hand stripping sticky Illinois ticktrefoil pods which tend to ball up in the combine (gloves and picking apron are shown; raincoat and rainpants, not shown, help to protect clothing). Hand harvesting or combinations of mechanical and hand harvest approaches may be needed for species with explosive seed capsules or capsules that shed seed immediately upon ripening. Strategies for collecting explosive seeds include: checking and harvesting daily (Viola), bagging seedheads loosely with a tightly woven mesh or cloth bag (Phlox), vacuuming seed from landscape fabric (Phlox), or clipping heads when some capsules have opened and drying cut material on tarps or cloth bags indoors (Ruellia). For the spiderworts (Tradescantia), check seedheads regularly near the typical harvest date. They begin shedding seed from the bottom of a flower cluster even when most of the plant is still green and there are still a few buds opening at the top of the cluster. When some seed is being shed from most plants, plots can be swathed and the cut material collected and laid on a tarp in a building. Capsules will continue to mature and release seed as the cut material dries.

Longbract spiderwort (Tradescantia bracteata) seed capsules mature and release seed even while other buds in a cluster are still flowering. Left: examining the underside of a seed cluster for open capsules. Center: hand cutting spiderwort stalks (the plot could also be swathed mechanically). Right: cut stalks drying in a building. The tarp catches the seed as the capsules mature and break open. Hand harvesting is also useful for collecting seed from wild populations for use as foundation material for seed increase and seed production plots. A seed collection is only a sample of the seed available in a population. How and when the seed is collected and propagated influences the genetic potential of the resulting population. Annual variation in rainfall and temperature can affect total seed production, quality, maturity, and dormancy. To optimize the capture of genetic diversity present in a population, approximately equal amounts of seed should be collected from many widely spaced individuals (minimum of 30, preferably 50 or more) throughout a site and over multiple years. In larger, evenly distributed populations, walk a linear transect through the population, sampling seed at intervals (e.g., every ten steps), then sample along additional transects parallel to the first. When collections from multiple sites are used to create a pooled population in a production plot, attempt to equalize the contribution of seed from each site.

Individual team members can collect along parallel transects to capture as much diversity as possible from a large plant population in a remnant prairie. Populations grown and re-grown in a production field can become adapted to site conditions and nursery management practices. Even though most prairie plants are perennial, seed production in plots tends to decline after 3-5 years for most species, and plots need to be replanted. Therefore, it is important to save seed from the original collections and/or the first production generation for replanting production fields. Hand harvesting of early maturing individuals in a production plot is a way to retain important genetic diversity within the production population. The rest of the plants in the production plot can then be mechanically harvested at peak maturity. Later maturing genotypes can be retained by leaving a portion of the plot uncut and harvesting it later (either mechanically or by hand).
- Mechanical Harvesting Techniques
Combine harvest
As stated above, not all seeds ripen at the same time; with any species, a determination has to be made as to when most seeds present are at or nearing maturity. Grasses are generally harvested at the hard dough stage with some exceptions. Test ripeness by firmly striking the seed head against your palm; if shattering occurs with only gentle striking, the stand should be combined immediately. Since most grasses ripen from the top down, some shattering of the tops of the seed heads may have already occurred. If a species’ seed shatters very easily, harvest in the early morning when humidity is high and wind speeds are less, as a strong wind can reduce the harvest significantly in a single afternoon. In grasses that shatter easily at maturity, windrowing or swathing during the medium- to hard-dough stage can be effective, since seed will after-ripen for several days after cutting. It is important to be sure no rains are in the forecast for the next few days after cutting. Swaths can then be picked up with a combine after the material has dried at the site for a few days.
Combines may require significant modifications to make them suitable for harvest of native grasses and forbs. Reducing or shutting off airflow in a combine is a must for native species with lightweight seeds. Species with fluffy pappus (e.g., asters and goldenrods) should be harvested when seed is mature but just prior to the dry-fluff stage. If the seed is dry-fluffy, the combine will become a super seed dispersal machine. Plugging of shaker sieves and augers is a constant issue, particularly with fluffy seed or seed with long awns. The long twisty awns of Canada wildrye can be a combine’s bane. De-awner bars can be installed into the cylinder surrounding the concaves to increase the threshing action of the concaves. Seed of tick trefoils (Desmodium species) is dispersed inside pods with sticky hairs. Some of the pods ball up in the combine, fail to pass through the sieves, and are ejected from the straw-walker. To prevent significant loss of seed, attach a bin or tarp to catch the seed balls. Alternatively, cut or swath the plot and collect the seed stalks, then pass them through a stationary combine, where the ejected material can be more easily captured.

Combining a plot of stiff goldenrod (Solidago rigida). For this and other fluffy seeded species, the airflow must be reduced or shut off. Seed strippers
Commercial seed strippers are available as handheld, implement pull-type, or tractor-mounted equipment. They all use a rotating brush or bristles to strip the seed from stems and stalks of plants. While perhaps not as efficient as combines, strippers can be used for the successive harvest of species that ripen gradually or at different times. Handheld strippers and pull-types light enough to be pulled with an ATV allow harvest of otherwise inaccessible native sites.
Leaf blower vacuum units
A leaf blower-vac unit on the vacuum setting can be used to harvest fluffy seed such as species in the aster family that have a pappus (“parachute”). Some suggest that the impeller blades of the machine need to be removed to avoid damaging the seed. However, we carried out a trial using an unmodified blower/shredder vacuum to harvest seed of prairie groundsel (Packera plattensis) and observed no visible damage to the seeds (cypselae) under a dissecting microscope. Because vacuuming removes seeds at the dry-fluff stage, immature seed can remain on the plants to ripen for later harvest. This should increase the yield of viable seed from each plant and reduce the risk of unintentionally selecting for a narrower seed ripening window. An additional benefit of this harvest method is that it removes much of the pappus from the seed at the harvest step, reducing the time needed in the seed cleaning lab. It would be interesting to try scaling up this technique using a modified pull-behind leaf vacuum.
- Ethics of Harvesting from Remnant Sites
Repeated, annual harvest of seed from remnant prairies for the commercial market is not encouraged. First, seed production and quality from wild populations are not as high as from production plots, and second, it provides an incentive to manage remnants for maximal seed production. Manipulation of a remnant prairie to maximize seed production – such as whole-site, repeated annual burns, herbicide treatments, or fertilizing – is inappropriate and unethical. A remnant prairie is a diverse, biotic community (both above and below ground) of microbes, fungi, plants, and vertebrate and invertebrate animals interacting in complex relationships. Management applied indiscriminately and repeatedly is detrimental to some of these associations. Burning should be limited to a portion of a remnant any given year, and each portion should be burned on rotation and at different times of year, at varying intervals of time. Bulk harvest from remnants may be appropriate when the seed is intended for planting on adjacent or nearby land to buffer and expand the native prairie. Finally, mechanical harvest in remnant sites should include a careful inspection and cleaning of equipment (including vehicles) to avoid introducing exotic/invasive species that may lead to the degradation of the remnant.
Be mindful of other ethical considerations when collecting seed from prairie remnants. Federal and state endangered and threatened species cannot be collected without proper permits. Removal of any plant or plant part from preserves, natural areas, and parks may be restricted. Check with the proper agency before harvesting seed in these areas. Harvesting from roadsides may also be restricted in some states and counties. Contact the county engineer’s office or state department of transportation before harvesting from county and state roadsides. Obtain permission from the landowner before collecting seed on private property. Some seed producers lease native prairies to compensate landowners for excluding grazing over the summer so that seed can be harvested from the site.

Obtain proper permits and permissions before making collections on private or public lands. Determining which entity owns or manages a site can be challenging. In the case of Clay Prairie, the land is owned by UNI, but the county helps manage the site, and the prairie is part of the State Preserves system.
Seed Processing
Seed Processing sagemThis section is under construction and new material will be added as soon as it is complete.
Post-harvest processes include drying, pre-cleaning, cleaning, and proper seed storage. The extent of processing required after drying depends on the species, storage conditions, and intended seeding method. Knowledge, skill, and access to specialized equipment are necessary for some of the cleaning steps described, and these factors will determine the quality of the finished product. Most commercial producers of native seed clean seed to a very high quality of purity and germination.
As in other steps in native seed production, care must be taken to avoid selection and loss of genetic diversity in production populations. In seed processing, there is a risk of selecting against genes that influence seed traits. For example, if only average-sized seeds are kept and used for expanding or re-growing fields, genes for seeds that are smaller or larger than average can decline in the population within a few generations.
- Drying
Drying is a necessary step for proper seed cleaning and storage for nearly all prairie species. Seed should be dried immediately after harvesting to prevent fungal growth and decomposition. Plastic bags or airtight containers can be used for storage ONLY after the seed is properly dried and cleaned. Larger quantities of material require specially constructed bins with screened bottoms and a source of airflow up through the material. Smaller quantities can be collected in large 100% cotton muslin bags made to fit inside a 30-gal plastic trash bin. Large cotton laundry bags with drawstrings work well. Fill bags loosely with seedheads, tie closed, label, and place in drying bins. If the material associated with the seed is very green, as is the case with spiderwort (Tradescantia spp.), or is damp from a recent rain shower, spread the material out on tarps and position several box fans overhead, turning the seed frequently with pitchforks, rakes, or shovels. Drying may take several days to a few weeks, depending on quantity of material and drying conditions (ambient humidity).

Image 1: Bin suitable for drying seed in cloth bags. 
Image 2: Cut stalks of Tradescantia bracteata laid to dry on a tarp. - Pre-cleaning
Harvested material requires some degree of pre-cleaning to reduce bulk. The extent of pre-cleaning required depends on the method of seed harvest, intended storage period, and method of planting. Threshing, debearding/deawning, and brushing are considered pre-cleaning steps, because they are designed to prepare the seed for later cleaning processes by removing unnecessary appendages and improving seed flow.
Threshing
Threshing removes the seed from seedheads, one of the primary functions of a combine harvester. Hand-collected material can be threshed with a variety of machines, including hammer mills, huller/scarifiers, and brush machines. Some growers have adapted older combines (with cutter bars and reel removed for safety) for use as a stationary thresher of hand-collected material. A debearder (see below) is effective for removing the awns of grass seeds and threshing the dried seedheads of compassplant and dried milkweed pods.

Image 3: Adding dried milkweed pods to the hopper of the Westrup debearder. 
Image 4: Seed falls into a tub positioned below the debearder, mixed with chopped pods and fluff. Non-mechanized threshing can be accomplished by stomping on seedheads. Using large plastic tubs, place about a 2-in. layer of bulk material in the bottom and stomp on it with waffle-sole boots. Toe kicks to the corners of the tub help break up any stubborn seedheads. Stomped material is then screened through a coarse ½-in. or ¼-in. screen into a second tub. Continue in batches, returning any intact seedheads remaining to the stomping tub. This method is very effective on species of Baptisia, Silphium, Helianthus, Veronicastrum, and Rudbeckia. Echinacea tends to be stubborn and requires machine threshing, unless it is collected late in the season after the seedheads naturally begin to shed their seed.
Deawning/Debearding
Many species have seed appendages such as the awns or “beards” on grasses and pappus “parachutes” on seeds of asters, goldenrods, and other composites. Removing or minimizing these appendages reduces bulk and improves seed flow for further cleaning. This step requires specialized and costly equipment. Debearding machines consist of a rotating shaft with projecting metal bars housed in a chamber. As the chamber fills with seed, the bars work the seed against itself, breaking or rubbing off the awns. It is important to fill the chamber with the proper amount of seed: too little and it’s ineffective, too much and the seed can heat up and be damaged. A continuous flow debearder works well for larger quantities of seed, but batch debearders are available for smaller quantities. A small gallon-size huller/scarifier is useful for deawning small individual accessions of seed. This type of machine is very aggressive, and only a few seconds of treatment are typically needed.

Image 5: A debearder being used to improve the flowability of combine harvested big bluestem (Andropogon gerardii) before cleaning the seed. A brush machine is useful for removing the pappus from asters, goldenrods, and blazing stars, but this versatile machine has many uses. Its basic action is to use rotating brushes to rub seeds or seedheads over a cylindrical drum screen, or mantle. Mantles come in various screen sizes, and a variety of brush textures is available. The machine can also be used as a deawner or scarifier. It is effective in removing the “cotton” from thimbleweed (Anemone virginiana and A. cylindrica), threshing seed from hand-collected mint seedheads, removing pods from purple prairie clover and leadplant, and deawning smaller quantities of grasses. Heavy canvas beater bars can be installed in place of brushes for a hammer-mill-like effect for removing seed from tough legume pods (e.g., Amorpha spp., Dalea spp., and Astragalus spp.) and dried rosehips (Rosa spp.). Beater bars are constructed from rubber/canvas multi-ply conveyer belting which can be purchased from industrial supply companies and cut to fit. When setting up the machine, position the beaters so that the canvas is barely touching the edge of the screen.

Image 6: A laboratory scale debearder with the front cover removed to show the screen mantle and rotating brushes. 
Image 7: A student worker feeding dried rosehips into the brush machine which has been set up with canvas beater bars. 
Image 8: The brush machine with beaters installed crushes the rosehips and releases the seeds. - Cleaning
- Storage
Seed Source and Quality
Seed Source and Quality sagemThis section is under construction and new material will be added as soon as it is complete.
- Importance of Seed Source
- Local Ecotype vs. Regional Sources
- Source-Identified Seed
- Cultivated Varieties of Native Species
- Seed Quality
- Calculating Pure Live Seed
Species Production Guides
Species Production Guides sagemThis section consists of specific information on seed production for selected species in the following functional groups: forbs (wildflowers), legumes, warm season grasses, cool season grasses, sedges, and woody species (prairie shrubs). Each species production guide includes a description of the species (with photos), its habitat, range map, and conservation status.
Recommendations for stand establishment, management, seed harvest, and seed cleaning are provided. These are based primarily on direct experience at the Tallgrass Prairie Center, together with published information from the NRCS Plant Materials Program, scientific publications, personal communication with other native seed growers, and publicly available databases such as the Seed Information Database and the Propagation Protocols Database of the Native Plant Network. A graph of potential seed yields is provided in each guide, based on harvest records from seed production plots at the Tallgrass Prairie Center.
Each species guide is provided as both a webpage and a printable file (pdf). If you prefer to work with a print version, we encourage you to print pdfs of the species you need and organize them in a binder.
This section is a work in progress. We will continue to add new species guides as they are completed. If you have comments, questions or would like to share propagation information for potential inclusion in these guides, please email laura.walter@uni.edu.
Forbs
Forbs sagemThe Species Production Guides for forbs (wildflowers) provide specific information about growing each of these species for seed production, based primarily on experience in growing them at the Tallgrass Prairie Center. The reference section in each guide provides links to other information sources. If you have information about producing seed of these species that you are willing to share, please feel free to contact us.
Scroll the list (alphabetized by scientific name) or press ctrl-f (or command-f) to search for any name in this page.
A printable file (pdf) is also provided on each species page for those needing a print version.
We will continue to add new species guides as they are completed.
Anemone cylindrica / candle anemone
Anemone virginiana / tall thimbleweed
Asclepias syriaca / common milkweed
Chelone glabra / white turtlehead
Coreopsis tripteris / tall tickseed
Potentilla arguta / tall cinquefoil
Euphorbia corollata / flowering spurge
Euthamia graminifolia / flat-top goldentop
Gentiana alba / plain gentian
Helianthus pauciflorus / stiff sunflower
Hypericum ascyron / great St. Johnswort
Liatris ligulistylis / Rocky Mountain blazing star
Monarda fistulosa / wild bergamot
Oligoneuron riddellii / Riddell's goldenrod
Parthenium integrifolium / wild quinine
Prunella vulgaris ssp. lanceolata / lance selfheal
Pycnanthemum verticillatum var. pilosum / whorled mountainmint
Silphium integrifolium / wholeleaf rosinweed
Solidago missouriensis / Missouri goldenrod
Symphyotrichum laeve / smooth blue aster
Symphyotrichum oolentangiense / skyblue aster
Symphyotrichum praealtum / willowleaf aster
Vernonia baldwinii / Baldwin's ironweed
Vernonia fasciculata / prairie ironweed
Zizia aurea / golden zizia
American water horehound
American water horehound dickeye
Lycopus americanus Muhl. ex W.P.C. Barton
Alternate Common Names: American bugleweed, cut-leaved bugleweed
Scientific Synonyms: Lycopus americanus Muhl. ex W.P.C. Barton var. longii Benner, Lycopus americanus Muhl. ex W.P.C. Barton var. scabrifolius Fernald, Lycopus sinuatus Elliott
Family: Mint family (Lamiaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with sprawling stems when not supported by neighboring plants, spreading by rhizomes to form loose to dense colonies.
Height: 1 - 2.5 ft

- Leaves and stem

Leaves opposite, larger and more deeply lobed near the base of the plant, nearly hairless, no minty scent when crushed, often turning purplish red in fall; stem four-angled, grooved, and mostly hairless, typically unbranched or with few branches.
- Flower, fruit and seedhead
Flower: Small (1/8 in) white flowers in dense clusters at leaf axils, calyx (sepals) fused into a tube with 5 triangular lobes; flower clusters bloom from the bottom to the top of the plant over the long flowering period.
Fruit/seed head: Four nutlets form in each calyx tube; nutlets are shorter than the calyx lobe, helping to distinguish this species from northern bugleweed (L. uniflorus).
Pollination: Small bees, wasps, flies, and other insects.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 155,000 (Prairie Moon Nursery)
1000 seed weight: 0.14 g (Seed Information Database)
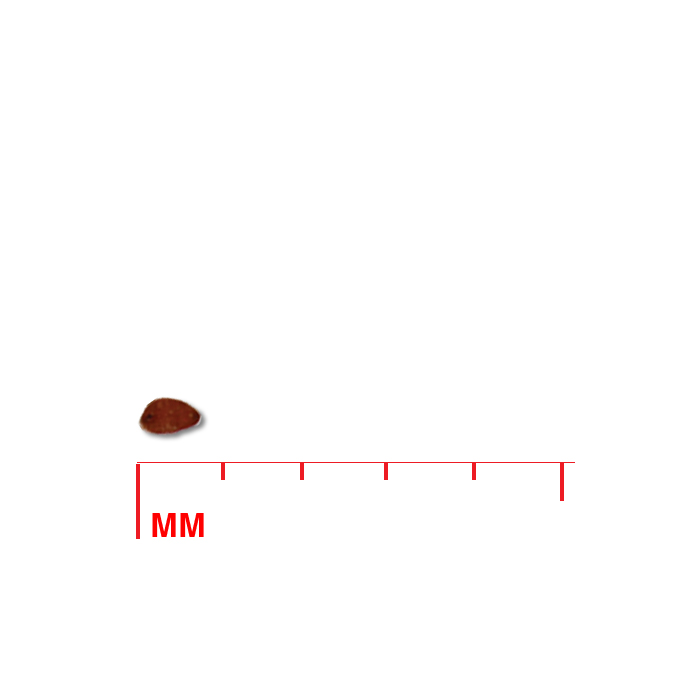
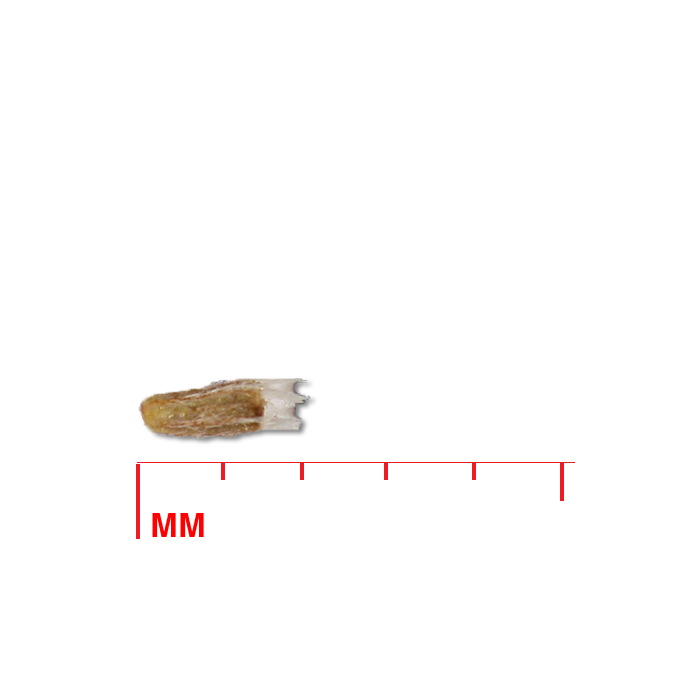
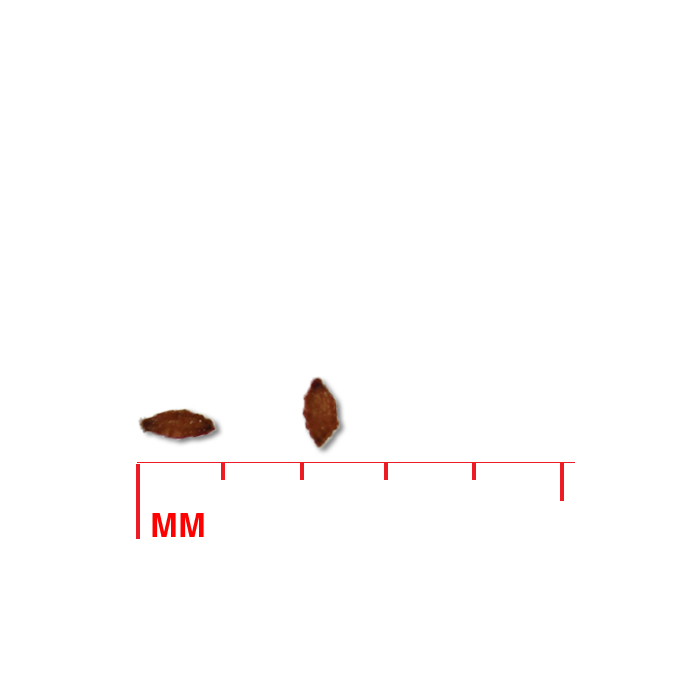
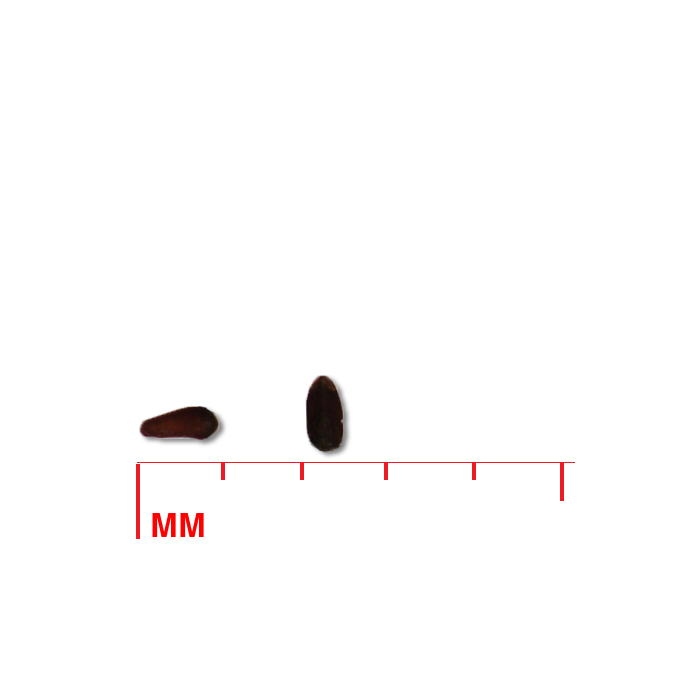
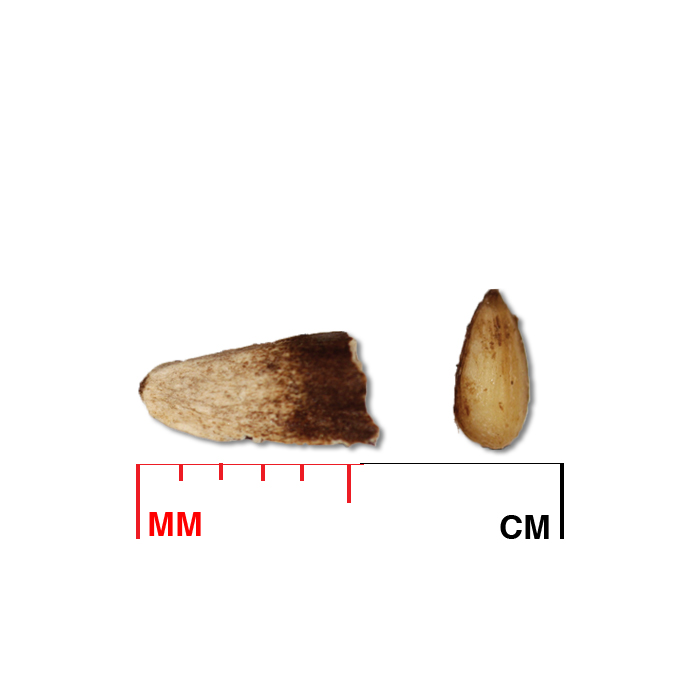
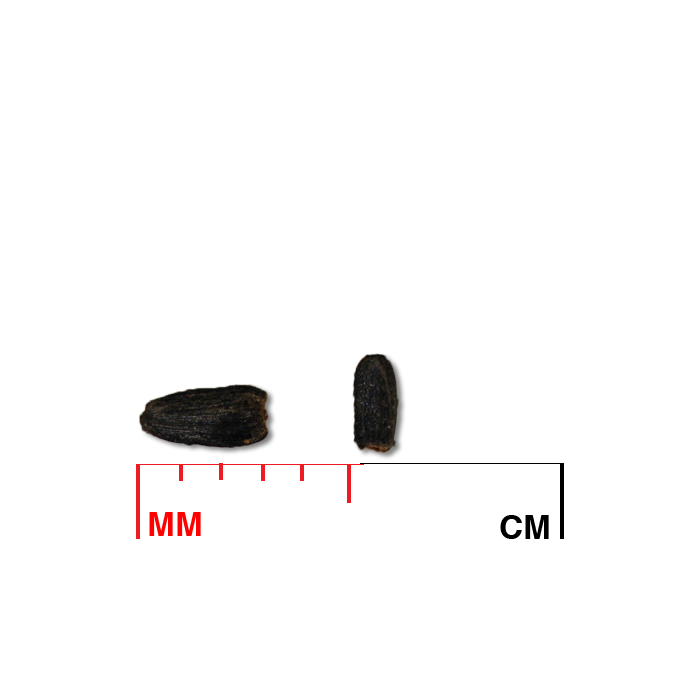
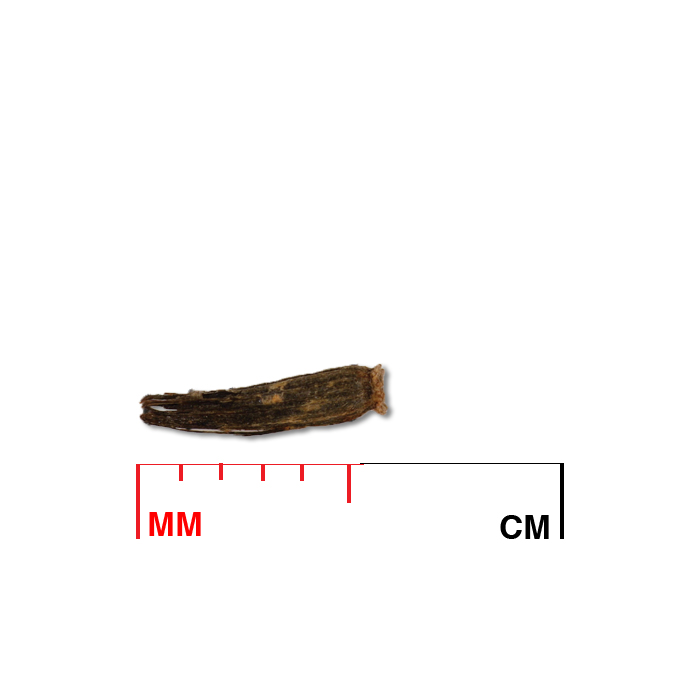
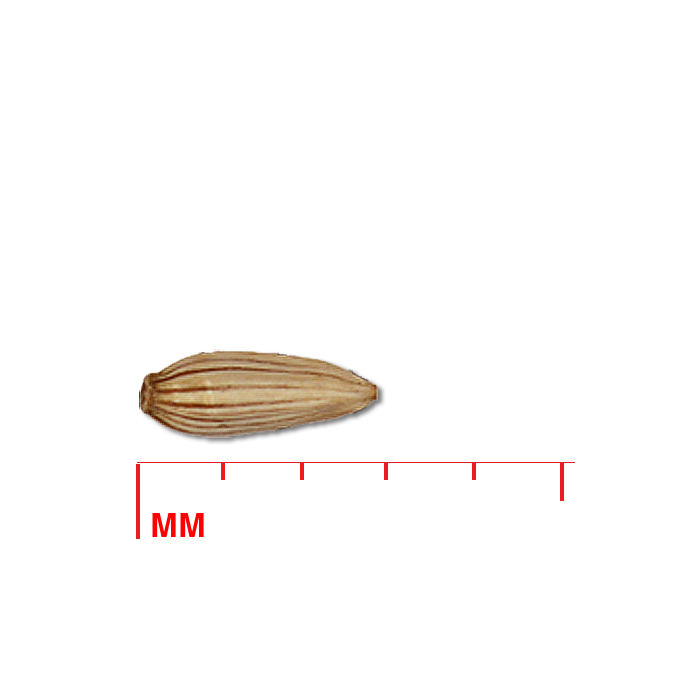
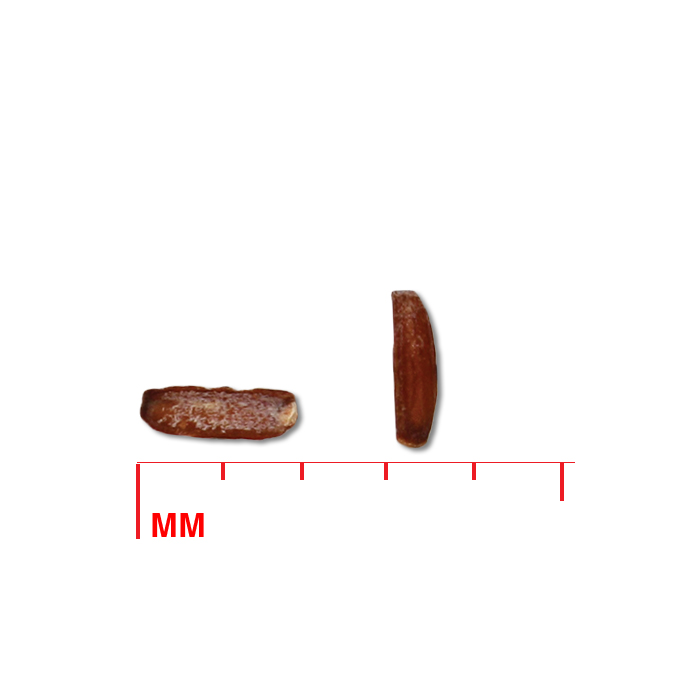
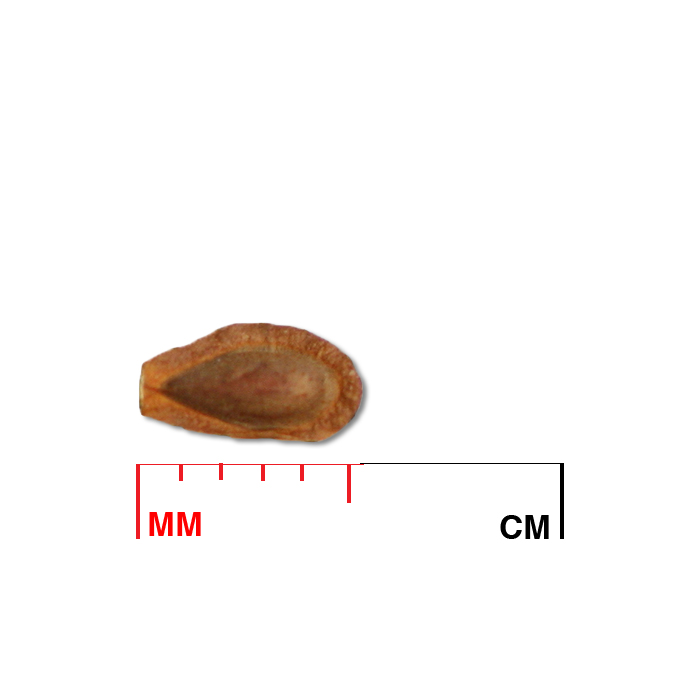
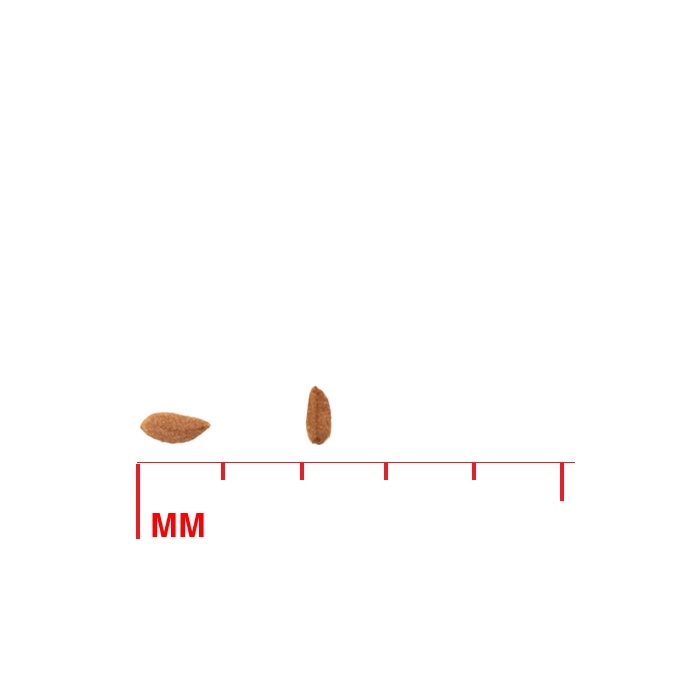
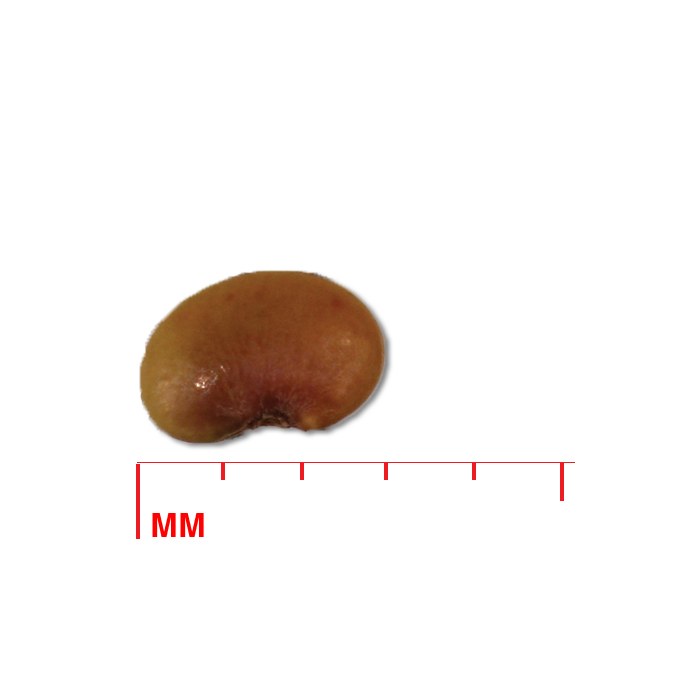
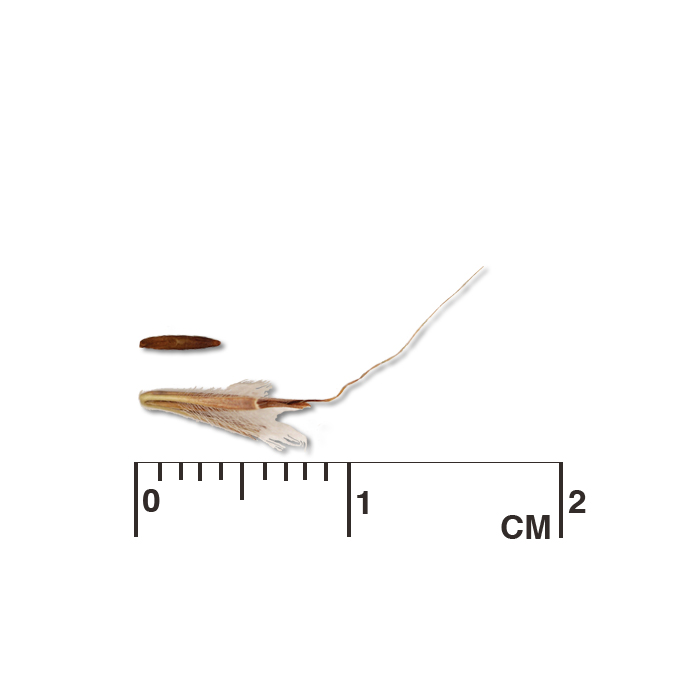
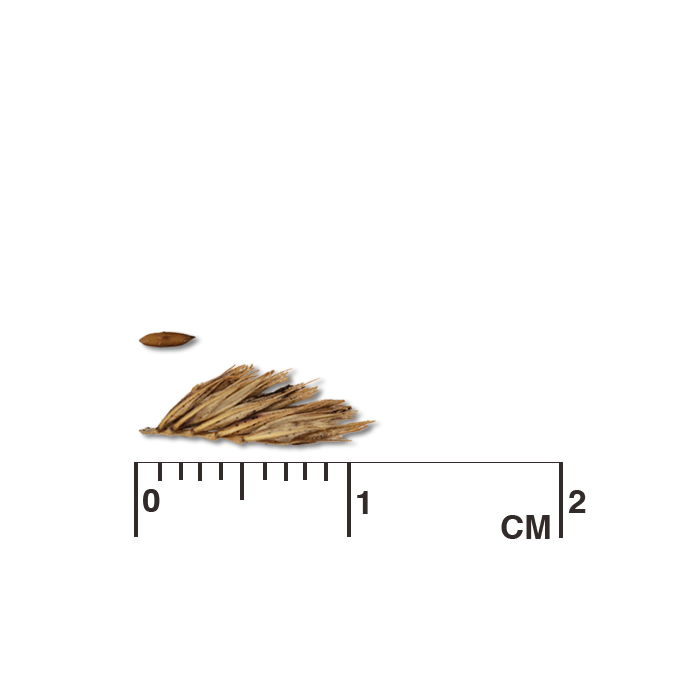
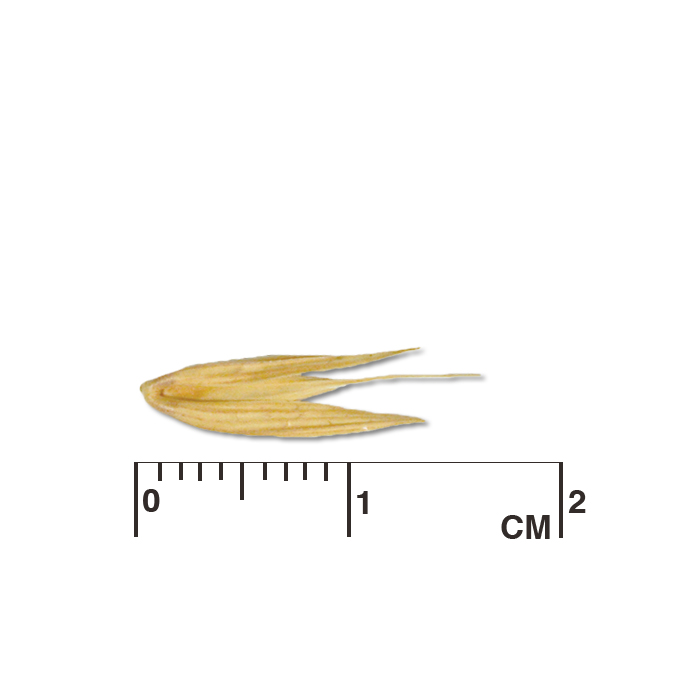
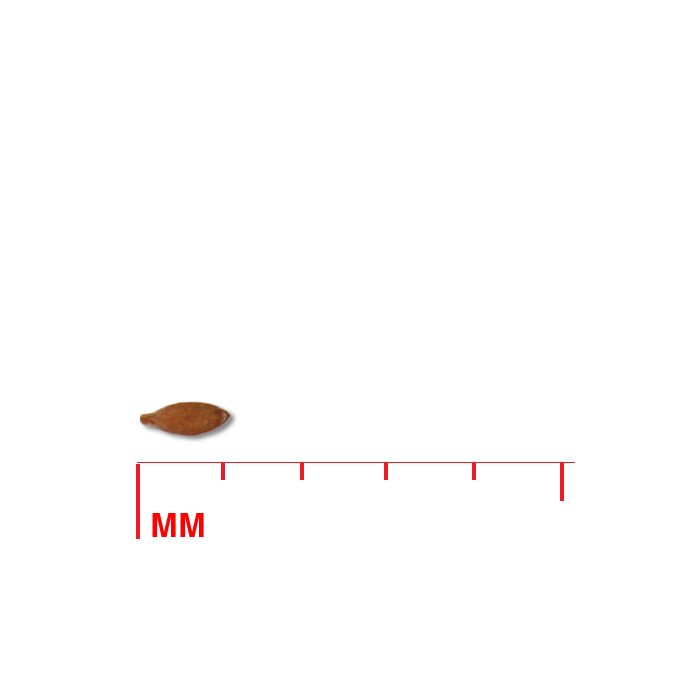
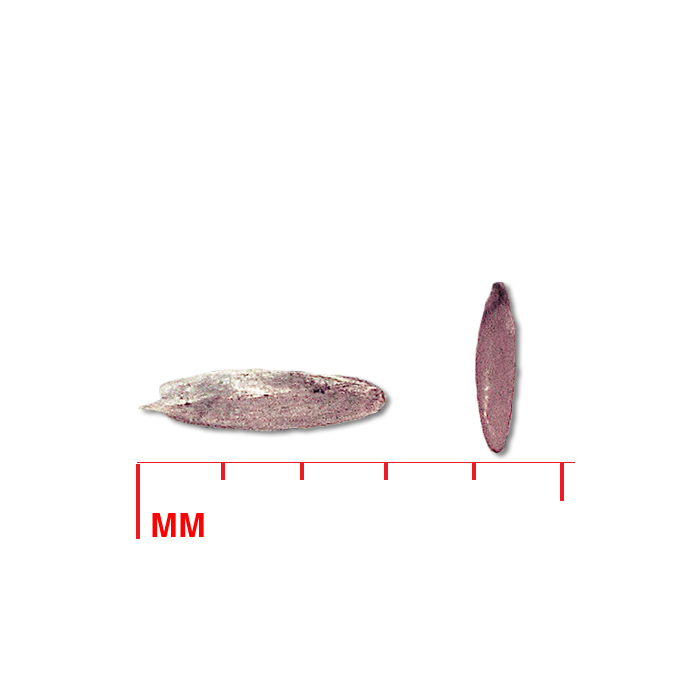
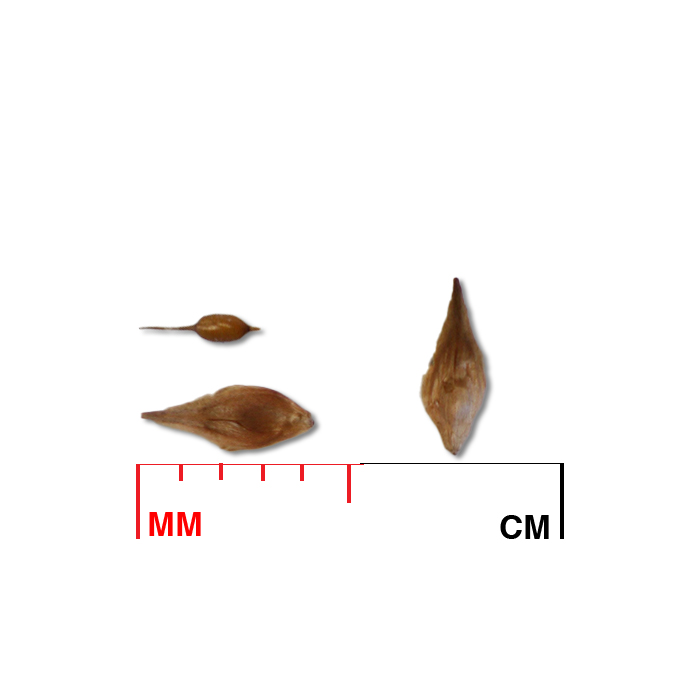
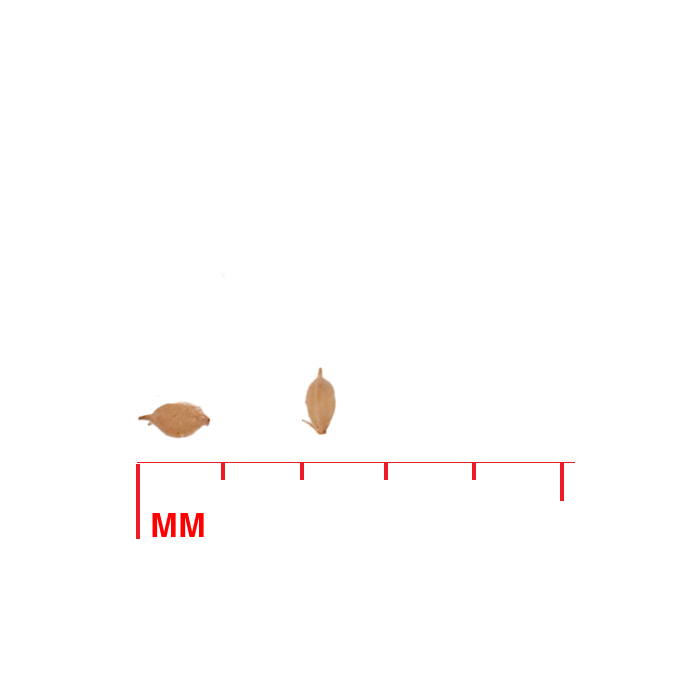
Description: Seed unit is a wedge-shaped, medium-brown nutlet, about 1 mm long, with clear beads of resin on one surface. Markings in the seed photo show millimeters.
Typical seed test
PLS: 73.8% (n=4)
Purity: 81.5% (n=4)
Germination: 2% (n=2)
Dormancy: 40% (n=2)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soils, full sun to partial shade; disturbed to high quality wet prairies, fens, marshes, shores, ditches; Wetland Indicator Status is OBL (obligate, almost always found in wetlands); irrigation is needed for seed production.
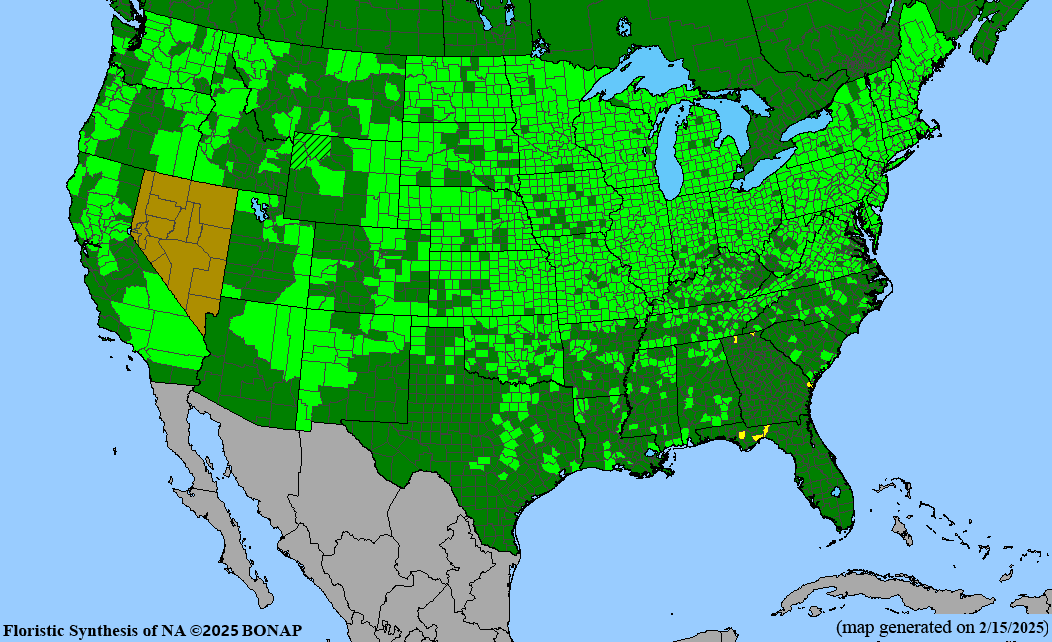
Conservation status: Global- G5 (secure); Colorado- S3 (vulnerable); North Carolina, South Carolina- S2 (imperiled); Alaska, Georgia- S1 (critically imperiled); in all other states, status is S4 (apparently secure) to S5 (secure) or unranked.
General Comments
American water horehound is not a particularly showy plant, but its long flowering time provides nectar and pollen resources for small bees, wasps, flies, and other pollinators for much of the summer into early fall. This species is found in both high quality and more disturbed remnant habitats in our region. While collecting this species for development of Iowa Source Identified stock seed, we found American water horehound in nearly every remnant wet prairie, sedge meadow, fen, or marsh we visited. This species even persists in some sites that are now dominated by cattails and reed canary grass. This species grows quickly from plugs in irrigated production rows and is productive in the establishment year. The vegetative spread of the plants produces a dense, leafy canopy that excludes many weeds, and harvesting and cleaning the seed is uncomplicated.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: 60 days cold/moist stratification.
Sowing: Surface sow in greenhouse about 2 months before last frost. Use caution when watering to avoid splashing out small seeds. Most seeds will germinate within two weeks of sowing and grow vigorously.
Transplanting: When plugs are well rooted, move them outside to harden off, then transplant into irrigated rows with plastic mulch at 8-12 in spacing.
- Stand management
Weeds: Prepare a clean, weed-free bed. Plastic mulch suppresses weeds in the first growing season but should then be removed to allow vegetative spread of plants. Plants grow densely and exclude many weeds. Mow or cultivate between rows. Hand rogue small seeded weeds that could contaminate the seed.
Pests: None noted.
Diseases: None noted.
- Seed production
 First Harvest: Plants grow rapidly, flower and set seed in their first growing season from transplants.
First Harvest: Plants grow rapidly, flower and set seed in their first growing season from transplants.Yield: 480 - 600 pounds/acre (extrapolated from harvests of one plot)
Stand Life: The first year’s harvest may be the highest yielding. We observed slightly lower yields in the second year and will continue to track yields for another year or two.
Flowering Date: late June - early Sept
Seed maturity/Harvest date: mid to late October
Seed retention: low risk of shattering
Harvest date range at TPC (2024-2025): Oct 20 - Oct 21
Recommended Harvest Method: combine (may need fairly high threshing speed)
- Seed cleaning and storage
Cleaning process: Pass combined material through 1/2 in and 1/4 in mesh to remove larger debris, then airscreen 2-3 times.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI (aligned with the Generalized Provisional Seed Zones of the US Forest Service)
- References
Bower, Andrew D.; St.Clair, J. Bradley; Erickson, Vicky. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Chayka, K. (n.d.). Lycopus americanus (American water horehound). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/american-water-horehound
Hilty, J. (2019). American bugleweed - Lycopus americanus. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/am_bugleweed.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: December 18, 2025).
Prairie Moon Nursery. (n.d.). Lycopus americanus. https://www.prairiemoon.com/lycopus-americanus-water-horehound
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
USDA NRCS National Plant Data Team. (n.d.). Lycopus americanus Muhl. ex W.P.C. Barton. USDA plants database. https://plants.usda.gov/plant-profile/LYAM
Species Guide Updated 12/19/2025
Canadian anemone
Canadian anemone dickeye
Anemone canadensis L.
Alternate Common Names: meadow anemone, Canada anemone, roundleaf thimbleweed, crowfoot, round-leaved anemone
Scientific Synonyms: Anemonidium canadense (L.) Á. Löve & D. Löve, Anemone dichotoma var. canadensis (L.) C. MacMillan
Family: buttercup family (Ranunculaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial, rhizomatous, forms colonies.
Height: 1-2 ft
- Leaves and stem

Leaves basal with long (6 in) stalks, roughly round in outline with 3-5 deep lobes, irregularly toothed margins, and flattened hairs; flowering stem is stiff and hairy and bears a whorl of three stalkless leaves.
- Flower, fruit and seedhead
Flower: Large white flowers, 5-parted, with numerous yellow stamens and a green center, up to 1 1/2 in wide, borne singly or in loose clusters of up to 3 flowers at tops of stalks.
Fruit/seedhead: Seed head mace-like, globular, containing numerous beaked achenes, often overtopped by foliage at maturity.
Pollination: Small bees and flies.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 8,000 (IA NRCS)
Seeds per pound: 128,000 (IA NRCS)
1000 seed weight: 2.89 g (Seed Information Database)
Description: ‘Seeds’ are actually one-seeded flattened fruits (achenes), about 1/8 in diameter, arranged in a ball-shape about 3/8 in diameter.
Typical seed test
PLS: 88% (n = 11)
Purity: 96% (n = 11)
Germination: 2% (n = 6)
Dormant: 91% (n = 6)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to wet-mesic soil; partial to full sun; moist prairies, sedge meadows, openings in floodplain woodlands, woodland borders, banks of streams, swampy areas; Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest.
Conservation status: Global- G5, secure; Kentucky and Tennessee- SX, presumably extirpated; District of Columbia, Maryland, and Virginia- SH, possibly extirpated; New Jersey and West Virginia- S1, critically imperiled; Connecticut and Wyoming- S2, imperiled; Kansas- S3, vulnerable (NatureServe)

General Comments
Canada anemone blooms early in the growing season, making it an important option for inclusion in pollinator habitat seed mixes. Remnant populations of this species persist along rural roads in Iowa, suggesting that it could be a long-lasting addition to roadside plantings, once established. Its low growth form, adaptability, and spreading habit may also be suitable for novel planting situations such as beneath solar panels. This species is challenging to propagate from seed because of its deep dormancy and difficulties with germination, but once established it tends to spread prolifically and is relatively easy to manage, harvest, and clean. Canada anemone may also be propagated by division of the rhizomes, but more than one genetic clone is needed for seed production.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
Seeding rate: 4.5 PLS pounds/acre (40 seeds/linear foot)
Seeding method: Native seed drill.
Seeding time: Dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Moist stratify 12 weeks at 40° F, or alternatively in ambient winter conditions (unheated building).
Sowing: Sow seed in greenhouse two months before last frost free date. Typically this species exhibits very high dormancy (low germination) and may require two winter cycles to germinate.
Transplanting: Transplant into bare soil in rows or weed barrier at 8 in intervals after all danger of frost is past. Once plants are established they spread prolifically by rhizomes, so the weed barrier will need to be removed or slit open to accommodate growth and enhance seed production.
- Stand management
Weeds: Post-emergent grass herbicide, tillage, hand roguing. Weed control is critical to successful establishment and seed production of this species. Read and follow label instructions.
Pests: Blister beetles may forage voraciously on foliage.
Diseases: None noted.
- Seed production
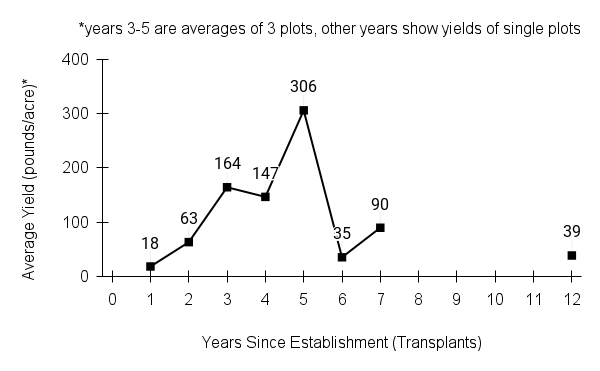
First harvest: Some flowering and seed set at the end of first growing season from greenhouse grown transplants. Direct seeded stands may take 3 years to become productive.
Yield: 18-306 pounds/acre (yields extrapolated based on production from 3 plots)
Stand life: Peak harvests occurred in the years 3-5 after transplanting. Stands at TPC have persisted for 20 years without management, but harvests made from one plot in years 7 and 12 were much smaller than peak yields.
Flowering date: mid-May - late June.
Seed maturity/Harvest date: mid - late July
Seed retention: Shattering occurs soon after (and perhaps before) seed maturity.
Harvest date range at TPC (2003-2015): July 15 - July 29
Recommended harvest method: Combine near maturity, but before seed head breaks apart.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping thru 1/2 in and 1/4 in mesh to remove large particles. Air-screen to clean.
Seed storage: Stores well in refrigerated conditions (32-40° F, 40-60% RH).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zones 1, 2, 3
- References
Chayka, K. (n.d.). Anemone canadensis (Canada anemone). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/canada-anemone
Dutton, B. E., Keener, C. S., & Ford, B. A. (2020, November 5). Anemone canadensis Linnaeus. Flora of North America. https://floranorthamerica.org/Anemone_canadensis
Hilty, J. (2019). Meadow anemone - Anemone canadensis. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/mdw_anemone.html
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 26–27). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Canada anemone. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 54–55). University of Iowa Press.
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
USDA NRCS National Plant Data Team. (n.d.). Anemone canadensis L.. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ANCA8
Species Guide Updated 12/1/2025
Culver's root
Culver's root dickeye
Veronicastrum virginicum, (L.) Farw.
Alternate Common Names: blackroot, Bowman’s root, Culver’s physic, high veronica, tall speedwell
Scientific Synonyms: Leptandra virginica (L.) Nutt., Veronica virginica L.
Family: figwort and snapdragon family (Scrophulariaceae); some authorities place this genus within the plantain family (Plantaginaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from a central taproot with side branches, spreading to form loose clumps by rhizomes.
Height: 3-6 ft

- Leaves and stem

Leaves whorled at nodes in groups of 3-7, up to 6 in long and 1 1/2 in] wide, lance-shaped with a finely serrated margin and sometimes hairy underneath; stem is smooth and round.
- Flower, fruit and seedhead
Flower: Inflorescence includes a central spikelike raceme up to 10 in long surrounded by one or more whorls of smaller spikes; each spike contains numerous, small (1/4 in), white, tubular flowers with yellow to pink-tipped stamens that extend out of the floral tubes; flowers bloom from the bottom to top in each spike.
Fruit/seed head: Inflorescence turns black-brown at maturity; fruit is an egg-shaped, two-celled capsule containing numerous tiny, reddish-black seeds.
Pollination: Insects, especially bees of diverse kinds.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 800,000 seeds/oz (IA NRCS)
1000 seed weight: 0.07g (Seed Information Database)
Description: Seed is dustlike; individual seeds are tiny (less than 1 mm long), egg-shaped, and dark reddish brown.
Typical seed test
PLS: 90% (n = 12)
Purity: 94% (n = 12)
Germination: 21% (n = 8)
Dormancy: 77% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to moist soil; partial to full sun; sand prairies, woodland openings and edges, swampy meadows, ditches, savannas, thickets; Wetland Indicator Status is Facultative (FAC) for the Midwest; wet-mesic, loamy soil is recommended for seed production, and irrigation may be beneficial, but avoid planting in areas where soil may be saturated for weeks at a time.
Conservation status: Global- G4, apparently secure; Louisiana and North Dakota- SH, possibly extirpated; Alabama, Delaware, District of Columbia, Nebraska, Oklahoma, South Carolina, South Dakota, and Vermont- S1, critically imperiled; Mississippi- S1/S2, critically imperiled to imperiled; Massachusetts, New York, and North Carolina- S2, imperiled; Kansas and Georgia- S3, vulnerable; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
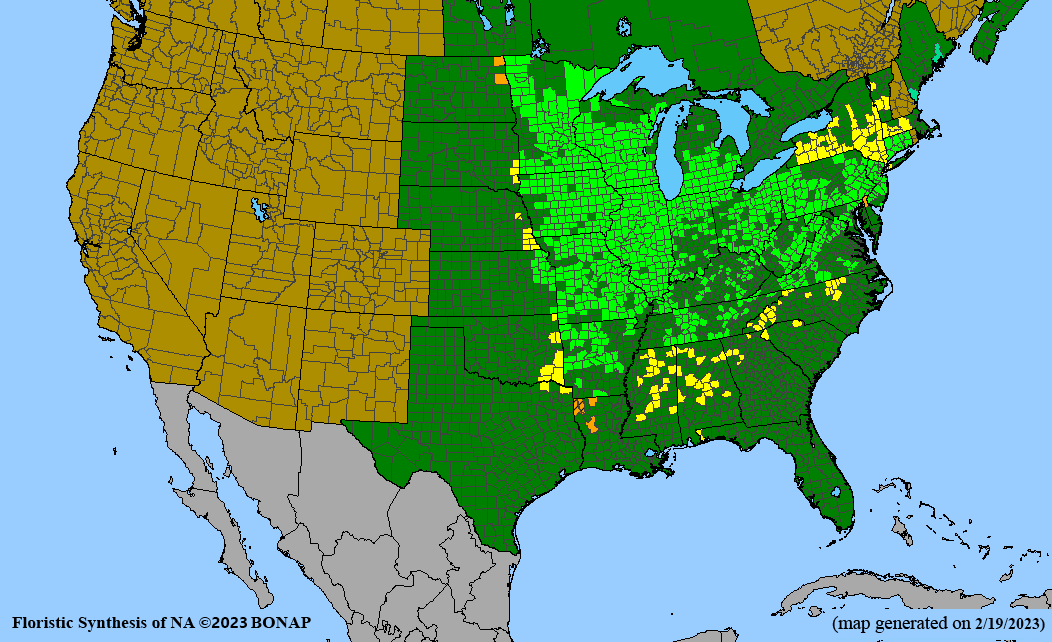
General Comments
Culver’s root apparently gets its common name from a pioneer doctor who prescribed it for various ailments, but it was already used by Native tribes for treating a variety of conditions. The whorled leaves and candelabra-like flower spikes make this species easy to identify. In our experience, it is shorter-lived in single-species production rows than in mixed plantings or native prairies, where individual plants may thrive for many years.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: Cold/moist stratification for 84 days.
Sowing: Surface sow in the greenhouse about 2-3 months before the last frost. Use caution when watering to avoid splashing seed from soil.
Transplanting: When seedlings have formed well-rooted plugs, move them outside to harden off, then transplant at 12 in intervals into beds prepared with plastic mulch.
- Stand management
Weeds: Prepare clean, weed-free beds. Plastic mulch suppresses weeds in the first year. Mow or cultivate between rows. Hand weed or rogue large, competitive weeds.
Pests: None noted.
Diseases: No specific diseases identified, but plants perform poorly if soil remains heavily saturated for several weeks as happened to one of our fields during a wet spring.
- Seed production
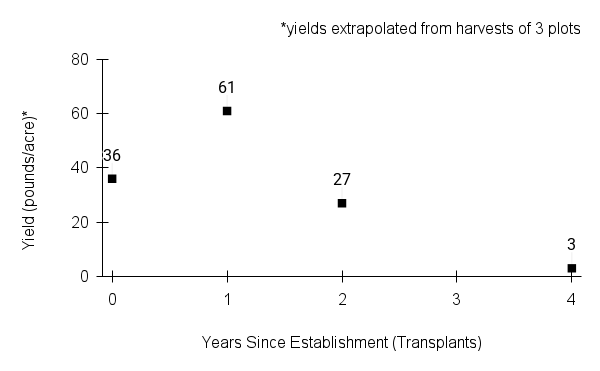 First harvest: Plants flower and set seed in the establishment year.
First harvest: Plants flower and set seed in the establishment year.Yield/acre: 20-60 pounds per acre (extrapolated from harvests of three plots)
Stand life: Peak yield may occur in second year, declining thereafter.
Flowering date: Late June through early August in northeast Iowa
Seed maturity/Harvest date: Late August to early October in northeast Iowa (may be delayed in the establishment year)
Seed retention: High risk of shattering; seed shakes out of capsules as they open.
Harvest date range at TPC (2003-2023): July 17 - November 2
Recommended harvest method: Hand-clip or combine when most inflorescences have turned brown and some capsules have opened.
- Seed cleaning and storage
Cleaning process: Brush hand collected material with medium bristles to thresh seed from capsules. Pass combined material through 1/4 in mesh to remove larger particles. Air-screen repeatedly.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern Iowa), Zone 2 (central Iowa), and Zone 3 (southern Iowa)
Cultivated varieties (cultivars): Selections have been made for the horticultural trade.
- References
Chayka, K. (n.d.). Veronicastrum virginicum (Culver’s root). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/culvers-root
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Culver’s-root. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 330). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Culver’s root - Veronicastrum virginicum. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/culverx.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Culver’s root. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 212–213). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Veronicastrum virginicum (L.) Farw. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=VEVI4
Species Guide Updated 12/29/2025
New England aster
New England aster dickeye
Symphyotrichum novae-angliae (L.) Nelson
Alternate Common Names: New England American-aster, purple meadow aster
Scientific Synonyms: Aster novae-angliae L., Lasallea novae-angliae (L.) Semple & L. Brouillet, Virgulus novae-angliae (L.) Reveal & Keener
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from short thick rhizomes.
Height: 3-4 ft
- Leaves and stem

Leaves alternate with leaf bases clasping the stem, softly hairy on the underside, closely spaced and persistent near the top of the plant; stem is hairy, reddish brown in color, mostly unbranched except within the inflorescence.
- Flower, fruit and seedhead
Flower: Composite flowerheads 1 to 1 1/2 in across with purple rays (occasionally pink or white) and yellow disk flowers, with long, spreading bracts (phyllaries) on the underside of each head. A single stem may bear 30-50 flowerheads in a panicle-like cluster.
Fruit/seedhead: Small hairy “seed” (achene) with a tuft (pappus) of light brown hairs. Seeds are wind-dispersed fairly soon after the pappus expands.
Pollination: Insects, primarily bees, flower flies, and butterflies.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 66,000 (IA NRCS)
1000 seed weight: 0.66 g (Seed Information Database)
Description: ‘Seeds’ are fruits 1/16 in long (achenes), with hairs attached to all parts of the seed coat.
Typical seed test
PLS: 86% (n = 10)
Purity: 92% (n = 10)
Germination: 37% (n = 9)
Dormant: 45% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Wet-mesic soil conditions, prairie swales, wet meadows, full sun; Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest. Avoid poorly drained clay soils for seed production purposes.
Conservation status: Global- G5, secure; Oklahoma- SH, possibly extirpated; Georgia, South Carolina, and Wyoming- S1, critically imperiled; Colorado- S2, imperiled; Kansas and North Carolina- S3, vulnerable (NatureServe)
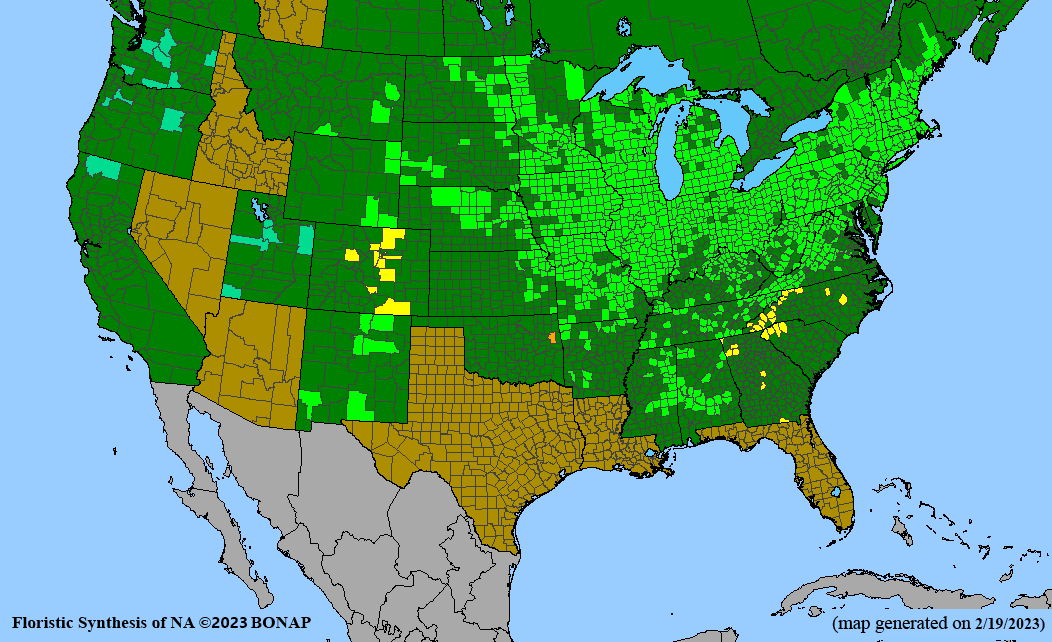
General Comments
This species is easy to propagate in the greenhouse. Though this species establishes readily in prairie reconstructions, weedy competition will severely curtail establishment and seed yield for seed production purposes. Fine hairs arise from nearly the entire surface of the seed, requiring thorough brushing/debearding to remove for good airscreen separation.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species
Greenhouse
Seed pre-treatment: Wet stratify 8 weeks at 40° F.
Sowing: Sow seed in the greenhouse 2 months before the last frost-free date.
Transplanting: Harden-off, transplant into bare soil in rows or weed barrier at 8-12 in intervals after all danger of frost.
- Stand management
Weeds: Post emergence grass herbicide, tillage, hand roguing. Weed pressure severely curtails establishment and seed production of this species.
Pests: Rabbits and other mammalian herbivores seem to favor eating foliage of this and other aster species and will keep plants pruned to 8 in throughout the growing season, particularly in the establishment year.
Diseases: Powdery mildew.
- Seed production
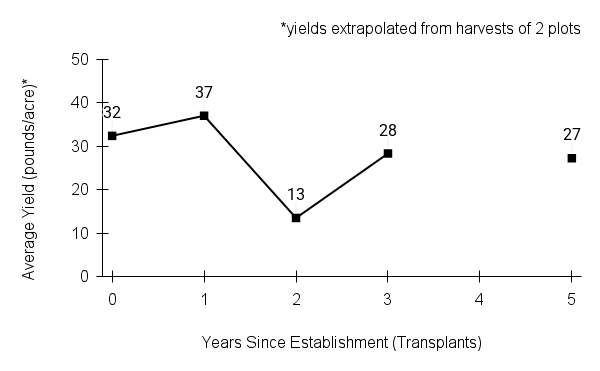 First harvest: Flowering and seed set at the end of first growing season from greenhouse grown transplants.
First harvest: Flowering and seed set at the end of first growing season from greenhouse grown transplants.Yield: 20-40 bulk pounds/acre (per acre yield extrapolated based on harvests from 2 plots)
Stand life: Peak harvests 1st and 2nd year. Seed production declines significantly 3rd year and after. For ornamental purposes, plants of this species are commonly pinched back through mid-summer to increase bushiness and flowering. Whether this would also increase seed production has not been demonstrated.
Flowering date: Late August - September in northern Iowa
Seed maturity/Harvest date: Mid-September - October in northern Iowa
Seed retention: Seeds are wind dispersed very soon after maturity.
Harvest date range at TPC (2004-2009): Oct 6-25
Recommended harvest method: Combine after seed maturity but before more than 10% of the seed heads have turned brown and fluffy. Otherwise, combining will simply contribute to dispersal of the seed crop. Harvested material will have to be forced-air dried and turned carefully to prevent mold and decay.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping through 1/2 in and 1/4 in mesh to remove large particles. Remove plumes with a debearder or brush machine, then air-screen.
Seed storage: cool/dry (32-40 F, 40-60% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Central Iowa Germplasm (IA Zone 2), Northern Iowa Germplasm (IA Zone 1), Southern Iowa Germplasm (IA Zone 3)
Cultivated variety (cultivars): Horticultural varieties also exist.
- References
Chayka, K. (n.d.). Symphyotrichum novae-angliae (New England aster). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/new-england-aster
Hilty, J. (2019). New England aster - Symphyotrichum novae-angliae. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/ne_asterx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 52–53). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Symphyotrichum novae-angliae (L.) G.L. Nesom. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SYNO2
Species Guide Updated 12/10/2025
Stiff tickseed
Stiff tickseed dickeye
Coreopsis palmata Nutt.
Alternate Common Names: stiff coreopsis, prairie coreopsis, prairie tickseed, finger tickseed, tickseed
Family: aster or sunflower family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial, fibrous-rooted and rhizomatous, spreads vegetatively to form clonal colonies.
Height: 1-3 ft
- Leaves and stem

Leaves opposite, sessile on the stem, deeply divided into 3 finger-like lobes; stem is rigid and grooved, mostly hairless except at the nodes, dark green in color, leafy, typically unbranched.
- Flower, fruit and seedhead
Flower: Yellow composite flower heads 1-2 in across with yellow centers that turn brown as they mature, ringed by 8-12 notched “petals” (ray florets); one to a few flower heads per stalk.
Fruit/seedhead: Center disk of head turns dark brown-black at maturity; only marginal flowers produce “seeds” (achenes).
Pollination: Insects such as bees, moths, butterflies, flies, and beetles.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 10,000 (IA NRCS)
Seeds per pound: 160,000 (IA NRCS)
1000 seed weight: 2.13 g (Seed Information Database)
Description: Seed units are oblong, flattened, inwardly curved achenes with winged margins, 1/4 in (5 mm) long.
Typical seed test
PLS: 64% (n = 11)
Purity: 70% (n = 11)
Germination: 41% (n = 10)
Dormant: 51% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to dry-mesic soil; full sun; medium to high quality prairie and savannas. For seed production, avoid wet or poorly drained soils.
Conservation status: Global- G5, secure; Louisiana and Michigan- S2, imperiled; Nebraska- S1/S3, critically imperiled to vulnerable; South Dakota- S3, vulnerable (NatureServe)
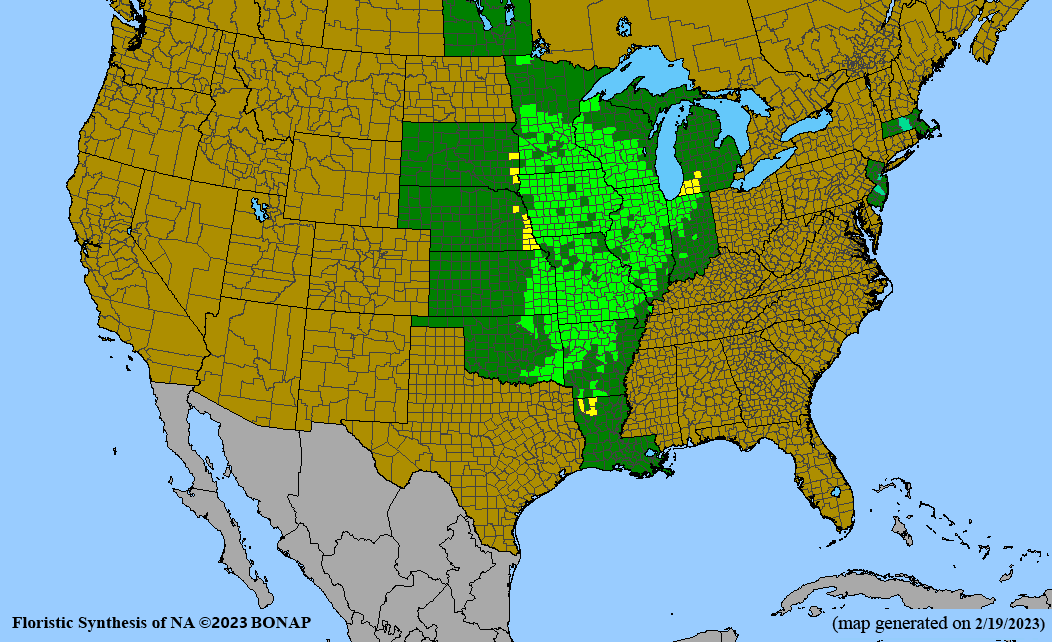
General Comments
Stiff tickseed typically occurs in colonies (clonal) in native prairies, spreading from rhizomes. This species supports numerous species of pollinators, including a specialist bee (Melissodes coreopsis), and herbivorous insects. Flattened, inwardly curved, winged achenes make air-screen separation of “seed” from inert plant parts difficult and this is reflected in the average purity reported from seed tests.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 3.0
Seeds per linear foot: 40
Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Wet stratify 8-12 weeks at 40° F.
Sowing: Sow seed in greenhouse at 1/4 in depth two months before last frost free date.
Transplanting: Harden off, transplant into prepared rows after all danger of frost. Permanent weed barrier is NOT recommended since this species spreads vegetatively, however, a biodegradable barrier can help reduce weed pressure during the first year. If planted into non-biodegradable plastic, holes must be opened further or plastic removed entirely in subsequent years.
- Stand management
Weeds: Weed barrier in first year can suppress many weeds. Mow or cultivate between rows. Consider post emergence grass herbicide and roguing to prevent weed seed from contaminating seed lots.
Pests: None noted.
Diseases: Crown root rot if planted in too wet soils.
- Seed production
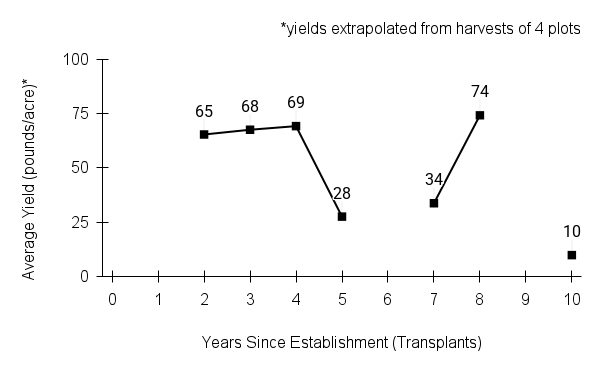 First harvest: Some flowering in the first growing season from transplants, but minimal seed production. Flowering and seed production increases in the second year.
First harvest: Some flowering in the first growing season from transplants, but minimal seed production. Flowering and seed production increases in the second year.Yield: 10-80 bulk pounds/acre (per acre yields extrapolated based on production from 4 plots)
Stand Life: Peak harvest appears to occur in the second to third year. Stand persists but seed production may decline or fluctuate in later years. Aerating the soil of the plot post-harvest with a turf aerator may enhance seed set the following season.
Flowering date: June - mid-July in northern Iowa
Seed maturity/Harvest date: October in northern Iowa
Seed retention: Relatively low risk; shattering occurs late October to early November
Harvest date range at TPC (2003-2021): Sept 11 - Nov 6
Recommended harvest method: Combine
- Seed cleaning and storage
Cleaning process: Pre-clean combined material by scalping through 1/2 in and 1/4 in mesh to remove large plant matter and brush (stiff bristles) to make flowable, then air-screen repeatedly, and indent if needed to remove small seeded weeds. Because seeds are flat, separation from leaf particles of similar size and weight requires repeated air-screen cleaning to improve purity. (No awns or appendages to remove.)
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zones 1, 2, and 3, Northern Missouri Germplasm, Western Missouri Germplasm
- References
Chayka, K. (n.d.). Coreopsis palmata (prairie coreopsis). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/prairie-coreopsis
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Prairie tickseed. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 73). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Praire coreopsis - Coreopsis palmata. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/pr_coreopsisx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 30–31). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Prairie coreopsis. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 124–125). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Coreopsis palmata Nutt. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=COPA10
Species Guide Updated 12/2/2024
Virginia mountain mint
Virginia mountain mint dickeye
Pycnanthemum virginianum (L.) T. Dur. & B.D. Jacks. Ex B.L. Rob. & Fernald
Alternate Common Names: common mountain mint, mountain mint, basil, mountain thyme, pennyroyal, prairie hysop
Scientific Synonyms: Koellia virginiana (L.) MacMill
Family: mint family (Lamiaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with short, slender rhizomes that forms vegetative colonies.
Height: 1-3 ft
- Leaves and stem
Leaves at least 3/16 in wide or more with a strong mint odor when crushed; stem is four-sided and smooth except for short hairs on the stem angles, often branched.
- Flower, fruit and seedhead
Flower: Small (1/8 in long) white, two-lipped flowers with light purple spots in tight clusters at branch tips, with many heads forming a flat-topped arrangement.
Fruit/seedhead: Mature seed heads are gray; four one-seeded nutlets develop inside each calyx tube.
Pollination: Insects such as bees, flies, wasps, and butterflies

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 220,000 (IA NRCS)
1000 seed weight: 0.14 g (Seed Information Database)
Description: ‘Seeds’ are nutlets, developing in a tube-like calyx of inflorescence.
Typical seed test
PLS: 87% (n = 11)
Purity: 93% (n = 11)
Germination: 48% (n = 8)
Dormant: 21% (n = 8)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Wet-mesic to mesic soils; low prairies; full sun; moist well-drained loamy soils preferred for seed production; moist sand prairies or meadows, swamps, thickets, rocky bluffs, fens; Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest.
Conservation status: Global- G5, secure; District of Columbia- SH, possibly extirpated; Alabama, Kansas, New Hampshire, and North Carolina- S1, critically imperiled; Arkansas- S1/S2, critically imperiled to imperiled; Georgia and Maryland- S2, imperiled; North Dakota- S3, vulnerable (NatureServe)
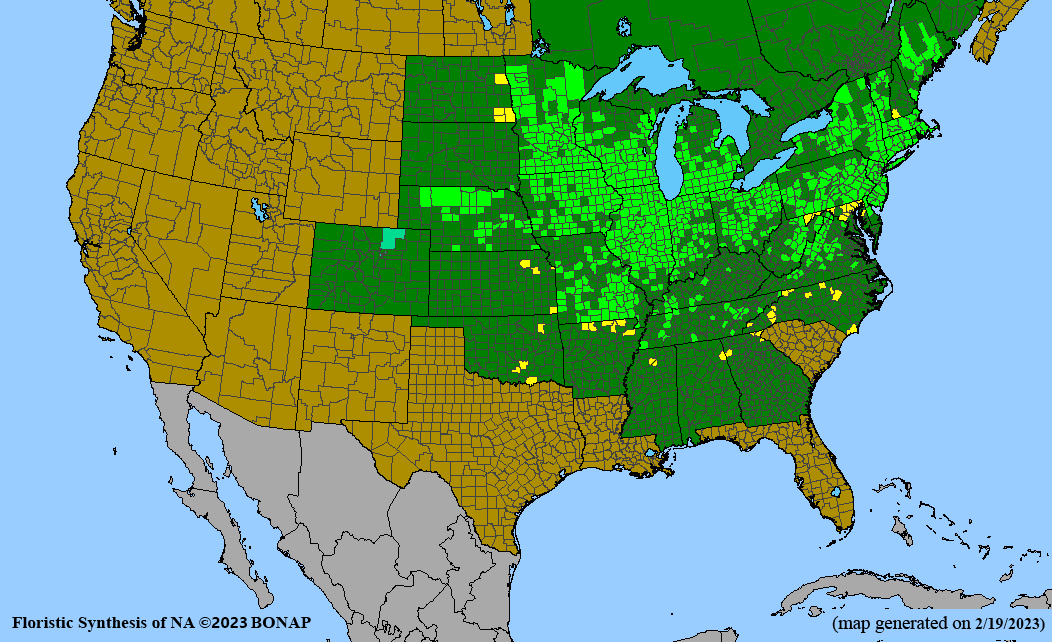
General Comments
This species is typically encountered (and noticed due to its minty smell when crushed) in patches or colonies in native prairie, spreading by rhizomes. Its gray seedheads and hairs on the stem angles help distinguish this species from narrowleaf mountainmint (P. tenuifolia). The small but abundant and long-lasting flowers of Virginia mountainmint attract numerous and diverse pollinators, particularly small bees, wasps, and beetles. Weed control is essential for good establishment and seed production. Can be harvested with a combine if plots are weed free. Seed is long-lasting under refrigeration.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: Dry cold stratify 12 weeks at 40° F.
Sowing: Surface sow seed in greenhouse two months before last frost free date. Water carefully (fine mist) to prevent seed from splattering out of containers.
Transplanting: Transplant into a weed barrier at 8 - 12 in intervals. Plants spread clonally, so the weed barrier can be removed by the third season, but seed production typically declines by the fourth season.
- Stand management
Weeds: Plastic mulch or weed barrier suppresses many weeds during the first year or two. Hand rogue weeds, being careful not to uproot seedlings or disturb roots and rhizomes of the mountainmint. An anecdotal report from a commercial native seed grower suggests that cultivation within mountainmint rows weakens plants and can cause loss of the crop.
Pests: None noted.
Diseases: None noted.
- Seed production
First harvest: Some flowering and seed production first year from greenhouse grown transplants.
Yield: 25-70 bulk pounds/acre in weed barrier, 15-45 bulk pounds/acre bare soil.
Stand life: Peak harvests second-third year. In proper soils with good management, stand and seed production persists at least into fourth year.
Flowering date: Flowering occurs mid-July into August.
Seed maturity/Harvest date: Mid September to early October.
Seed retention: Holds seed well, shattering occurs mid to late October.
Harvest date range at TPC (2003-2007): Sept 18 - Oct 21
Recommended harvest method: Combine
- Seed cleaning and storage
Cleaning process: Pre-clean by scalping through 1/2 in and 1/4 in mesh to remove large particles and make flowable, then air-screen. (No awns or appendages to remove).
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zones 1 (northern Iowa), 2 (central Iowa), and 3 (southern Iowa)
- References
Chayka, K. (n.d.). Pycnanthemum virginianum (Virginia mountain mint). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/virginia-mountain-mint
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Mountain mint. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 221). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Common mountain mint - Pycnanthemum virginianum. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/cmt_mintx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 46–47). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Virginia mountain mint. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 218–219). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Pycnanthemum virginianum (L.) T. Dur. & B.D. Jacks. ex B.L. Rob. & Fernald. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=PYVI
Species Guide Updated 12/8/2025
Virginia strawberry
Virginia strawberry dickeye
Fragaria virginiana Duchesne
Alternate Common Names: wild strawberry, common strawberry
Scientific Synonyms: Fragaria australis, Fragaria canadensis, Fragaria grayana, Fragaria terrae-novae
Family: rose family (Rosaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
perennial, spreading by runners to form extensive clones.
Height: 4-8 in

- Leaves and stem

leaves basal, palmately compound; oval shaped leaflets (3) are up to 2.5 in long and 1.5 in wide with coarsely toothed margins and often finely haired, especially on the undersides; stems are sprawling stolons, often reddish in color, that root at the tips where new plants can emerge.
- Flower, fruit and seedhead
Flower: regular, five-petaled, 1/2 in diameter, petals white, stamens and pistils in center of flower yellow, 4-6 flowers in a loose cluster that is usually shorter than the leaves.
Fruit/seedhead: 1/2 in globular to ovoid “berry” with numerous achenes (“seeds”) in pits on the berry’s surface, bright red at maturity.
Pollination: Insects such as bees, flies, and butterflies.


- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 83,000 (Prairie Moon)
1000 seed weight: 0.46g (Seed Information Database)
Description: “Seed” is a reddish-brown achene, 1.2-1.8 mm in diameter, roughly egg-shaped.
Typical seed test
PLS: 83.35%
Purity: 99.81%
Germination: 21%
Dormancy: 61%
TZ: 85%
(averages obtained from 1 test of a purchased seed lot, and 1 test of seed produced at TPC)
- Habitat and range
Habitat: Dry to moist soil; partial to full sun; prairies, meadows, woodland openings and edges, roadsides, along railroads, savannas, limestone glades; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest.
Conservation status: Global- G5, secure; Louisiana- S1, critically imperiled; Nevada- S2, imperiled; Illinois- S2/S3, imperiled to vulnerable (NatureServe)
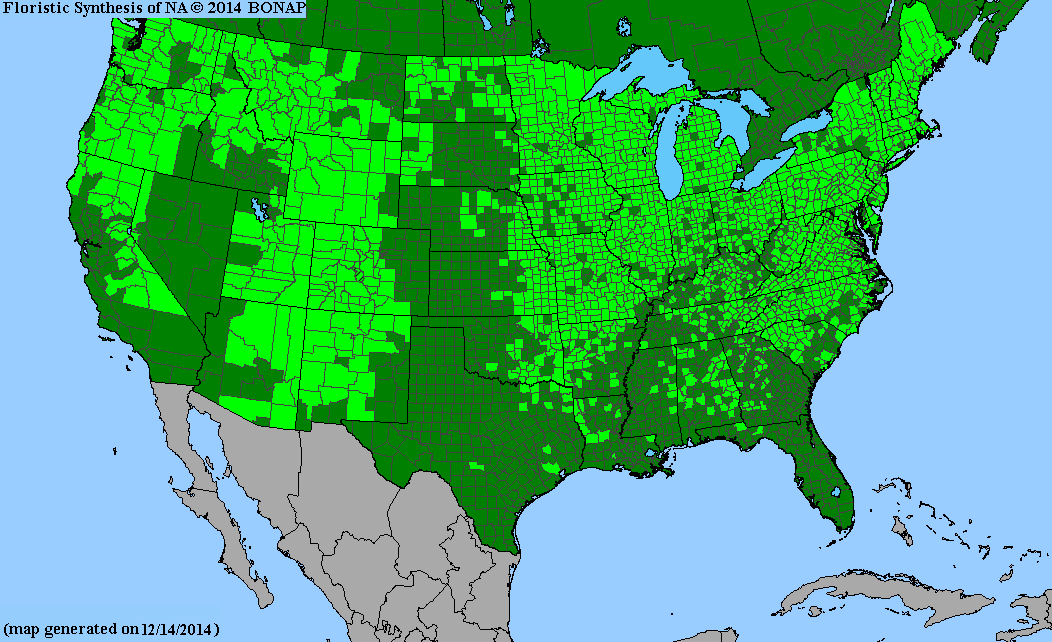
General Comments
Virginia strawberry can be found in the “understory” layer of most remnant prairies in Iowa. These small plants pack a large ecological punch. They flower early in the season, providing nectar and pollen resources for many species of small bees, flies, and skipper butterflies. Various herbivorous insects and mammals feed on the leaves, and the fruits are eaten by birds, mammals, and even reptiles (turtles), which disperse the seeds. When wild strawberries were abundant across the Iowa landscape prior to agricultural conversion, the fruits were an important early summer food for both Native people and Euro-American settlers. Virginia strawberry is one of the species that produced the cultivated strawberry through hybridization. Propagation and processing of this species is not difficult compared to other prairie forbs, and the most significant barrier to seed availability is probably the cost of labor required to pick the berries several times during the 2-3 week fruiting season.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: 8 weeks cold/moist stratification
Sowing: Sow seed in greenhouse 8-10 weeks before average frost free date; spread stratified seed on germination flats and lightly cover (1/8 in or less) with germination mix; move seedlings to plugs when they have a pair of well-developed true leaves.
Transplanting: When plugs are well rooted and danger of frost is past, harden off seedlings and transplant into weed-free beds prepared with biodegradable paper mulch weighted with clean straw. Mulch must be biodegradable so that the new plantlets that form at the ends of stolons can grow roots into the soil.
- Stand management
Weeds: Biodegradable mulch in the first growing season suppresses most weeds; mowing at the highest setting of a typical riding mower after the fruiting season keeps weeds from becoming overly competitive in the second year.
Pests: Mammalian herbivores may browse foliage, and birds and small mammals consume fruits.
Diseases: None noted, though diseases that affect commercially grown strawberries such as botrytis molds may become a problem.
Plot renewal: Commercially grown strawberries are known to produce more fruit at the edges of plots. Since wild strawberries are closely related to the cultivated varieties, they may behave similarly, and techniques of cultivation, fertilization, and irrigation that are used by commercial strawberry farms may be beneficial. We will update this information as we gain more experience.
- Seed production
First Harvest: Plants flower and set fruit in the second year after planting.
Yield/Acre: 17-18 lbs of seed per acre (extrapolated from first year’s production of one production plot); it takes roughly 30 pounds of berries to obtain a pound of seed.
Stand Life: Probably long-lived, but production may decline as plots grow densely in subsequent years; plants at the edges of clones may set more fruit than those within dense patches; techniques used for “renewing” cultivated strawberry production such as rototilling narrow strips through beds may be helpful.
Flowering Date: May in northern Iowa
Seed maturity/Harvest date: late May to mid June in northern Iowa
Seed retention: There is a risk of seed loss from animals consuming the fruit or from fungal diseases; during the fruiting season (2-3 weeks), berries must be picked every 2-3 days.
Harvest date range at TPC: May 26 to June 13 (first harvest, 2024)
Recommended Harvest Method: Hand pick every other day. The labor required to harvest the small berries is significant: it took over 40 person-hours to gather ripe berries from an 840 sq ft plot every few days over a 19 day period, yielding 11.52 lbs of fruit and 5.47 oz of clean seed.
- Seed cleaning and storage
Cleaning process: Place 2 parts water to 1 part fruit in a blender and process for 30 seconds. (Blender blades do not need to be altered or wrapped.) Pour the resulting mash into a 5 gallon bucket and add additional water. Stir, then allow the filled seed to settle out. Pour off the floating material, being careful to save the heavy seed at the bottom (J. Carstens, USDA-NCRPIS, personal communication, January 10, 2022). Spread the seed onto muslin cloth and place in front of a fan to dry. Airscreening will remove remaining debris.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI
The map below shows the collection locations for populations used in development of this ecotype on a base map of the Generalized Provisional Seed Transfer Zones of the US Forest Service.
- References
Chayka, Katy. (n.d.). Fragaria virginiana (wild strawberry). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/wild-strawberry
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Wild strawberry. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 301). University of Wisconsin-Madison Arboretum.
Gleason, H. A., & Cronquist, A. (1991). Rosaceae. In Manual of Vascular Plants of Northeastern United States and Adjacent Canada (2nd ed., p. 242). The New York Botanical Garden.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
Staudt, G. (2020, July 30). Fragaria virginiana (Miller). Flora of North America. http://dev.floranorthamerica.org/Fragaria_virginiana
University of Minnesota Extension. (2025). Strawberry Farming. https://extension.umn.edu/fruit-and-vegetable-farming/strawberry-farming
USDA NRCS National Plant Data Team. (n.d.). Fragaria virginiana Duchesne. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=FRVI
Species Guide Updated 2/3/2025
bluejacket
bluejacket dickeye
Tradescantia ohiensis Raf.
Alternate Common Names: Ohio spiderwort, common spiderwort, cow-slobbers, snotweed, smooth spiderwort
Scientific Synonyms: Tradescantia canaliculata Raf., Tradescantia foliosa Small, Tradescantia incarnata Small, Tradescantia ohiensis Raf. var. foliosa (Small) MacRoberts, Tradescantia reflexa Raf., Tradescantia barbata
Family: dayflower or spiderwort family (Commelinaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from fibrous, fleshy roots.
Height: 1.5 -2.5 ft

- Leaves and stem
Leaves smooth, grass-like and almost succulent with a waxy bluish-green sheen, alternate, joining main stem as a sheath, generally hairless at maturity although leaves of seedlings may have hairs; stem smooth, unbranched.
- Flower, fruit and seedhead
Flower: Clusters of few to many buds at stem tip and upper leaf axils; flower buds bent downwards within a cluster, bending upwards on smooth flowering stalks as each bud flowers; flowers with three blue-violet petals (occasionally white to light purple) and 6 yellow anthers with fine violet hairs at base; sepals smooth and hairless (helping distinguish this species from T. bracteata); each flower opens for a day, primarily in the morning hours.
Fruit/seedhead: Dark gray to black seeds develop inside three-parted capsules that split open and drop seed at maturity, starting at the base of a flower cluster.
Pollination: Insects, primarily bumblebees. Spiderworts produce pollen but no nectar.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 8,000 (IA NRCS)
Seeds per pound: 128,000 (IA NRCS)
1000 seed weight: 3.66 g (Seed Information Database)
Description: Seeds develop inside three-parted capsules that split open and drop seed at maturity. Seed coats are dark gray to black with intricate, wrinkled ornamentation.
Typical seed test
PLS: 91% (n = 11)
Purity: 98% (n = 10)
Germination: 6% (n = 7)
Dormant: 89% (n = 8)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic soils, prefers sandy soils in remnant prairies and open woodlands, often in areas with some disturbance; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; full sun and well-drained loam soils preferred for seed production.
Conservation status: Global- G5, secure; New Jersey- S2, imperiled. (NatureServe)
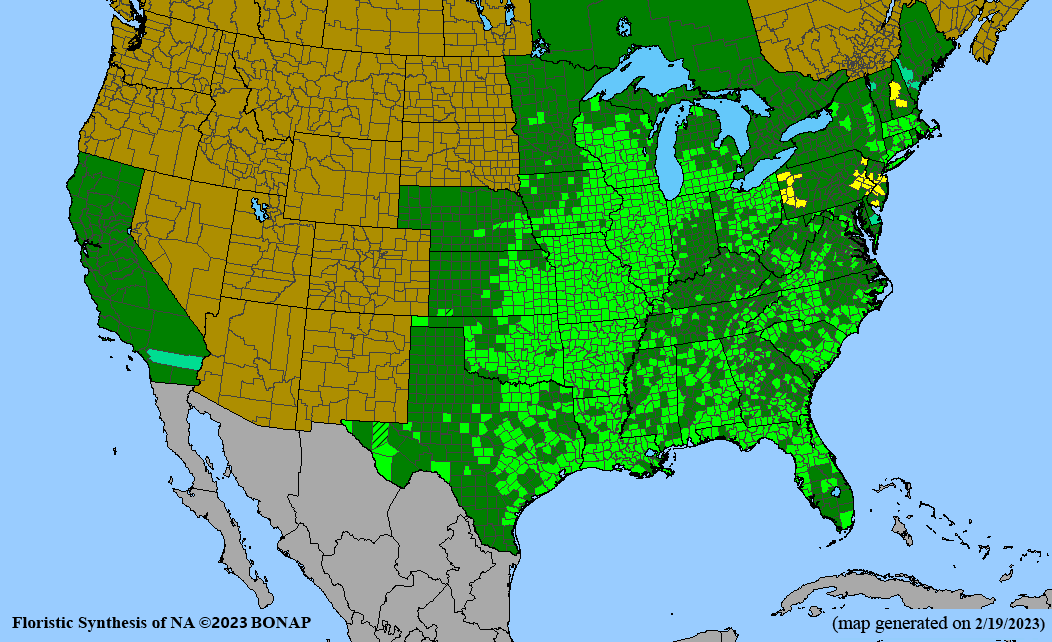
General Comments
This species is easily propagated in the greenhouse for transplanting into production beds. Plants establish readily in prairie restorations and will spread with good management. Timing of seed harvest is challenging, since flowering and seed maturity occur gradually, and the sepals in the flower clusters may still appear green and fleshy even after much of the seed has dropped. Also, plants have a slimy, sticky sap (hence the unglamorous but obvious common name ‘snotweed’), which makes direct combining inadvisable.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 4.5
Seeds per linear foot: 40
Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: High percentage of dormancy, seed must be wet stratified 12 weeks at 40°F.
Sowing: Sow seed at 1/4 in depth in greenhouse 2 months before the last frost-free date.
Transplanting: Transplant into bare soil at 30-36 in row spacing or into a weed barrier at 8-12 in spacing after all danger of frost is past.
- Stand management
Weeds: Post emergence grass herbicide, tillage, hand roguing.
Pests: None noted. Rabbits and deer will browse foliage.
Diseases: None noted.
- Seed production
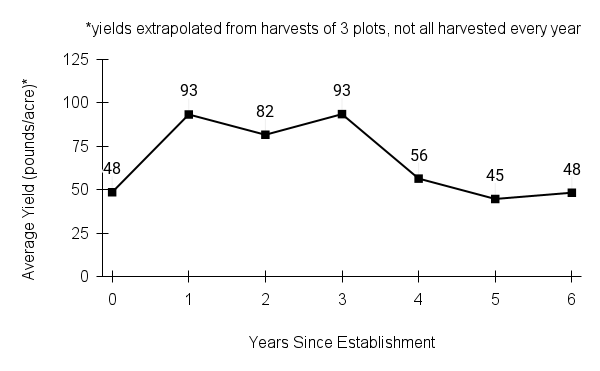 First harvest: Some flowering and seed set ( 24-48 bulk pounds/acre) at the end of the first growing season from greenhouse grown transplants.
First harvest: Some flowering and seed set ( 24-48 bulk pounds/acre) at the end of the first growing season from greenhouse grown transplants. Yield: 40-100 bulk pounds/acre (per acre yields extrapolated based on production from 3 plots, not all of which were harvested each year)
Stand life: Peak harvests in the second and third full growing season after establishment. Seed production declined somewhat 4th year and was about half peak harvest 5th year. Chisel plowing can reinvigorate stands. Spiderwort is reportedly tolerant of low rates (1%) of glyphosate.
Flowering date: Late May - late June in northern Iowa
Seed maturity/Harvest date: June - mid-July in northern Iowa
Seed retention: Shattering occurs as soon as seed ripens in each capsule within a cluster. Monitor plots frequently during the later part of the flowering season. Turn over flower clusters and observe for opened capsules. Aim to harvest when a few capsules are open on most heads even though some flowers may still be present. The color of sepals changes as the capsules mature; this is helpful but variable and not a consistent indicator of readiness.
Harvest date range at TPC (2003-2023): June 24 - July 23 (but first year stands from transplants may mature much later than is typical)
Recommended harvest method: Hand pick seed heads and dry on tarps for several days with good air circulation. If piles are thick, turn often to avoid mold. Most seed will be released from capsules as they dry, and threshing largely entails simply scalping off the dried vegetative material. Large fields may be machine swathed, but seed will shatter out of heads as material dries down.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping through 1/2 in and 1/4 in mesh to remove large particles, and then air-screen. Hard seed coats can visually mask seed quality. Aspiration (air screening) of seed is critical to remove unfilled but otherwise normal-looking seed.
Seed storage: cool/dry (50°F, 30% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern Iowa), Zone 2 (central Iowa), and Zone 3 (southern Iowa)
- References
Chayka, K. (n.d.). Tradescantia ohiensis (Ohio spiderwort). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/ohio-spiderwort
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Common spiderwort. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 155). University of Wisconsin-Madison Arboretum.
Gleason, H. A., & Cronquist, A. (1991). Commelinaceae. In Manual of Vascular Plants of Northeastern United States and Adjacent Canada (2nd ed., p. 655). The New York Botanical Garden.
Hilty, J. (2019). Ohio spiderwort - Tradescantia ohiensis. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/oh_spiderwortx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 54–55). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Tradescantia ohiensis Raf. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=TROH
Species Guide Updated 12/11/2025
butterfly milkweed
butterfly milkweed dickeye
Asclepias tuberosa L.
Alternate Common Names: butterfly weed, pleurisy root, yellow milkweed, orange swallowwort, orangeroot, whiteroot, Indian posy, windroot, Canada tuber, Canada flux, chigger flower
Family: milkweed family (Asclepiadaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with a woody taproot that forms elongated tubers, can form clumps of many stems but does not spread by rhizomes.
Height: 1-2.5 ft
- Leaves and stem
Alternate leaves are lance-shaped with entire margins and dense to scattered hairs, short stalked or nearly sessile on the stem; stems are visibly hairy, branched, green to reddish in color. Unlike other milkweeds, butterfly milkweed has no milky sap.
- Flower, fruit and seedhead
Flower: Orange (sometimes red or yellow) blooms, 5-parted, in flattened clusters of up to 25 flowers on tips of branches.
Fruit/seedhead: Seedpods (follicles), 4-6 in long, often in clusters, covered in peachlike fuzz, seeds bear a silky, white “parachute” and are distributed by the wind.
Pollination: Insects, particularly butterflies, bees, and wasps.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 4,300 (IA NRCS)
Seeds per pound: 68,000 (IA NRCS)
1000 seed weight: 6.76 g (Seed Information Database)
Description: Seeds are flat and chocolate-brown, about 1/4 in by 3/16 in with a tuft of fine filaments (floss).
Typical seed test
PLS: 93% (n = 12)
Purity: 100% (n = 12)
Germination: 63% (n = 8)
Dormant: 48% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic, well-drained soil; full sun; high quality remnant prairies, savannas, glades, roadsides.
Conservation status: Global- G5, secure; Maine- SX, presumably extirpated; Vermont- SH, possibly extirpated (NatureServe)

General Comments
The bright orange flowers make this a desirable species for horticultural displays as well as prairie reconstructions. This species, like other members of the genus Asclepias, is an important host plant for monarch butterfly caterpillars. Seeds germinate readily in the greenhouse with proper stratification or use of gibberellic acid. Seedlings require care in transplanting because of the taproot structure. Butterfly milkweed does best in very well-drained soils. Requires hand-harvesting as pods ripen.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 8.5 (40 seeds/linear foot)
Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Wet stratify 8 weeks at 40° F or treat for 24 hours with 250-ppm gibberellic acid.
Sowing: Sow seed in greenhouse two months before last frost free date. Susceptible to damping off in the greenhouse. Use sterile potting mix with added perlite, sanitize planting materials, and provide adequate ventilation. Also vulnerable to greenhouse pests such as thrips and aphids. If possible, empty and sanitize greenhouse between growing seasons to reduce pest pressure.
Transplanting: Plant into bare soil in 36 in rows or weed barrier at 12 in intervals.
- Stand management
Weeds: Mow or cultivate between rows and/or use a weed barrier. Post-emergence grass herbicide, tillage, and/or roguing may be used to control weeds. Always read and follow label instructions.
Pests: Yellow milkweed aphids on upper portions of plant, including pods. Light infestations may be controlled by naturally occurring aphid predators and parasites. Heavy infestations can cause abortion of pods and failure of crop. Monarch larvae eat foliage and pods, but not a serious problem on this milkweed species. Milkweed bug nymphs feed on developing seeds within pods. In small scale plantings, manual removal of clusters of bug nymphs can improve seed quantity and quality.
Diseases: Susceptible to various fungi, bacteria, a phytoplasma, and protozoan pathogens (Borders and Lee-Mäder, 2014).
Hybridization risk: Hybrids have been found between Asclepias tuberosa and some related species in the genus Asclepias, however it appears to be rare.
- Seed production
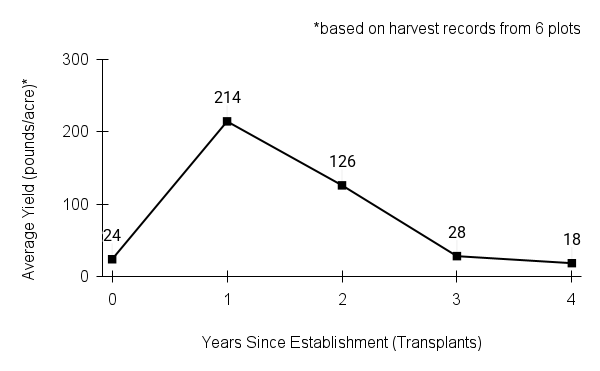 First harvest: Some flowering and minimal seed production is possible in the planting year with greenhouse transplants.
First harvest: Some flowering and minimal seed production is possible in the planting year with greenhouse transplants.Yield: 18-214 bulk pounds/acre (yields extrapolated based on production from 6 plots)
Stand life: Peak harvests are typically in the second year. Stand persists in well-drained soils if disease free, but seed production may decline significantly in subsequent years. Aeration of the soil may improve stand life and productivity.
Flowering date: mid-June - mid-August in northern Iowa
Seed maturity/Harvest date: mid-August - mid-October in northern Iowa
Seed retention: Seed dispersed by wind soon after pods split open.
Harvest date range at TPC (2003-2023): Aug 10 - Oct 14
Recommended harvest method: For small plots, check daily and hand harvest as pods ripen. Ripe pods usually have a blush of yellow (somewhat like a ripe peach) and split readily with an audible “pop” when gently squeezed at the “seam.” Seeds are mature if they appear chocolate brown. If they’re still creamy white, leave the pod unpicked for another day or two.
- Seed cleaning and storage
Cleaning process: Seeds can be separated from freshly picked pods with a hammer mill, or from dried pods using a debearder. Winnow the debearded material through a coarse screen on a day with a steady, gentle wind or in front of a box fan to reduce the bulk and fluff, then air-screen.
Seed storage: Stores well in refrigerated conditions (32-40° F, 40-60% RH).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zones 1, 2, and 3; Glacial Lake Albany Germplasm (NY)
Cultivated variety (cultivars): Horticultural varieties may also exist.
- References
Borders, B. & Lee-Mäder, E. (2014). Milkweeds, A Conservation Practitioners Guide. Xerces Society for Invertebrate Conservation. https://www.xerces.org/publications/guidelines/milkweeds-conservation-practitioners-guide
Chayka, K. (n.d.). Asclepias tuberosa (butterfly-weed). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/butterfly-weed
Hilty, J. (2019). Butterfly milkweed - Asclepias tuberosa. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/btf_milkweedx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 28–29). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Butterfly milkweed. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 128–129). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Asclepias tuberosa L.. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ASTU
Species Guide Updated 12/2/2024
button eryngo
button eryngo dickeye
Eryngium yuccifolium Michx.
Alternate Common Names: button snakeroot, rattlesnake master, rattlesnake-master, yucca-leaf eryngo, corn snakeroot, water-eryngo, rattlesnake flag, rattlesnake weed
Family: carrot family (Apiaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from a central taproot; forms small clumps through offsets after blooming.
Height: 2-5 ft
- Leaves and stem
Tough, fibrous, yucca-like leaves are mostly basal, with a waxy surface and widely spaced spiny teeth along margins; stem is hairless, waxy and rigid, pale bluish green in color.
- Flower, fruit and seedhead
Flower: Spherical flowerheads composed of numerous small white flowers are arranged on short branches on the upper portion of the plant.
Fruit/seedhead: Seed head with prickly bracts turns golden-tan by winter; mature seed heads are dark brown when wet.
Pollination: Insects, predominantly bees and wasps, but also butterflies, flies, moths, and beetles.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 7,500 (IA NRCS)
Seeds per pound: 120,000 (IA NRCS)
1000 seed weight: 3.82 g (Seed Information Database)
Description: Typical “seed units” are one-seeded scaly fruits, 3/16 in long.
Typical seed test
PLS: 93% (n = 10)
Purity: 98% (n = 9)
Germination: 19% (n = 8)
Dormant: 43% (n = 8)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry-mesic to wet-mesic soil; full sun; occurs in medium to high quality remnant prairies, savannas, and limestone glades; Wetland Indicator Status is Facultative (FAC) for the Midwest. Well-drained loamy soils preferred for seed production.
Conservation status: Global- G5, secure; Maryland- SH, possibly extirpated; Nebraska- S1, critically imperiled; Michigan and Virginia- S2, imperiled; Minnesota and Ohio- S3, vulnerable (NatureServe)

General Comments
Greenhouse propagation is recommended for this species. It grows readily, and produces some seed the first year from transplants. Potentially high seed yield. Fairly straightforward to combine harvest and air-screen clean.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 3.25
Seeds per linear foot: 40
Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: dormant season
Weed control: Prepare a clean, firm, weed free seed bed prior to seeding.
Greenhouse
Seed pre-treatment: Wet stratify 8-12 weeks at around 40° F.
Sowing: Sow seed (1/4 in deep) in greenhouse two months before last frost free date.
Transplanting: When roots are developed enough to produce a sturdy plug, harden off, then transplant into bare soil in rows or weed barrier at 8-12 in intervals after all danger of frost.
- Stand management
Weeds: Weed barrier or plastic mulch suppresses weeds during the first year. Mow/cultivate between rows. Post emergence grass herbicide, tillage, and/or hand roguing prevent weed seed contamination of crop. Anecdotal reports from commercial seed growers suggest that this species may persist and produce well within a matrix of grasses, including non-native cool season species, which may help suppress other weeds.
Pests: None noted.
Diseases: Cucumber mosaic virus detected in populations in Ohio (Whitten and Nameth, 2004).
- Seed production
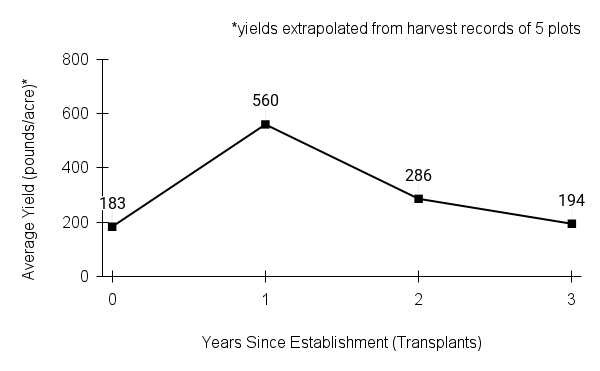 First harvest: Small harvest first growing season if greenhouse propagated in March and planted into a weed-barrier in spring. Flowering and harvest may be delayed in the planting year. First harvest in second year if direct seeded.
First harvest: Small harvest first growing season if greenhouse propagated in March and planted into a weed-barrier in spring. Flowering and harvest may be delayed in the planting year. First harvest in second year if direct seeded.Yield: 180-560 bulk pounds/acre (yields extrapolated from harvest records from 5 plots)
Stand life: Peak harvests in the second to third years. Stand declines fourth year.
Flowering date: mid July-mid August in northern Iowa
Seed maturity/Harvest date: early October in northern Iowa
Seed retention: Shattering occurs mid to late October
Harvest date range at TPC (2003-2023): Sept 7 - Oct 25
Recommended harvest method: combine
- Seed cleaning and storage
Cleaning process: Pre-clean combined material by scalping thru 1/2 ft and 1/4 in mesh to remove large particles and make flowable, then air-screen. (Brushing is not needed; no awns or appendages to remove.)
Seed storage: cool/dry (33-50° F, 30-50% RH).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Northern Iowa Germplasm (IA Zone 1), Central Iowa Germplasm (IA Zone 2), Southern Iowa Germplasm (IA Zone 3)
- References
Chayka, K. (n.d.). Eryngium yuccifolium (rattlesnake master). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/rattlesnake-master
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Rattlesnake-master. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 45). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Rattlesnake master - Eryngium yuccifolium. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/rattlesnakex.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 34–35). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Rattlesnake master. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 130–131). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Eryngium yuccifolium Michx. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ERYU
Species Guide Updated 12/4/2025
cardinalflower
cardinalflower dickeye
Lobelia cardinalis, L.
Alternate Common Names: bog sage, cardinal-flower, hog’s physic, Indian pink, red bay, scarlet lobelia, slinkweed, water gladiole
Scientific Synonyms: Lobelia cardinalis L. ssp. graminea (Lam.) McVaugh, Lobelia cardinalis L. var. graminea (Lam.) McVaugh, Lobelia cardinalis L. var. multiflora (Paxton) McVaugh, Lobelia cardinalis L. var. meridionalis Bowden, Lobelia cardinalis L. var. pseudosplendens McVaugh, Lobelia cardinalis L. var. phyllostachya (Engelm.) McVaugh, Lobelia cardinalis L. var. propinqua (Paxton) Bowden, Lobelia fulgens Humb. & Bonpl. ex Willd., Lobelia splendens Humb. & Bonpl. ex Willd.
Family: bellflower family (Campanulaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Short-lived perennial with fibrous root system that forms clumps of stems in the second year of growth.
Height: 1-5 ft

- Leaves and stem
Leaves alternate, lance-shaped, up to 6 in long, with a crinkled surface texture and coarsely toothed and somewhat wavy margins, the stem is ridged and mostly hairless, usually unbranched.
- Flower, fruit and seedhead
Flower: Flowers scarlet-red, 1-1.5 in long; tubular and two-lipped with the lower lip three-lobed and an erect, tubular extension of the corolla from which the stamens and stigma protrude; inflorescence of numerous flowers is a spike-like raceme up to 2 ft long.
Fruit/seed head: Two-parted capsules develop within the persistent calyx and withered corolla, opening at maturity to release numerous, tiny, golden seeds.
Pollination: Primarily hummingbirds and large butterflies.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 400,000 (IA NRCS)
1000 seed weight: 0.04g (Seed Information Database)
Description: Seeds are less than 1 mm long, translucent, honey-colored, and elliptical in outline with an intricate, bumpy surface texture.
Typical seed test
PLS: 95%
Purity: 97%
Germination: 4%
Dormancy: 70%
(averages obtained from 4 tests of purchased seed lots)
- Habitat and range
Habitat: Moist, sandy soil; part shade to full sun; wet meadows, woodland openings, shores; Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest. Irrigation is recommended for seed production.
Conservation status: Global- G5, secure; Nebraska- S1, critically imperiled; Nevada- S1/S2, Critically imperiled to imperiled; Colorado- S2, imperiled; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
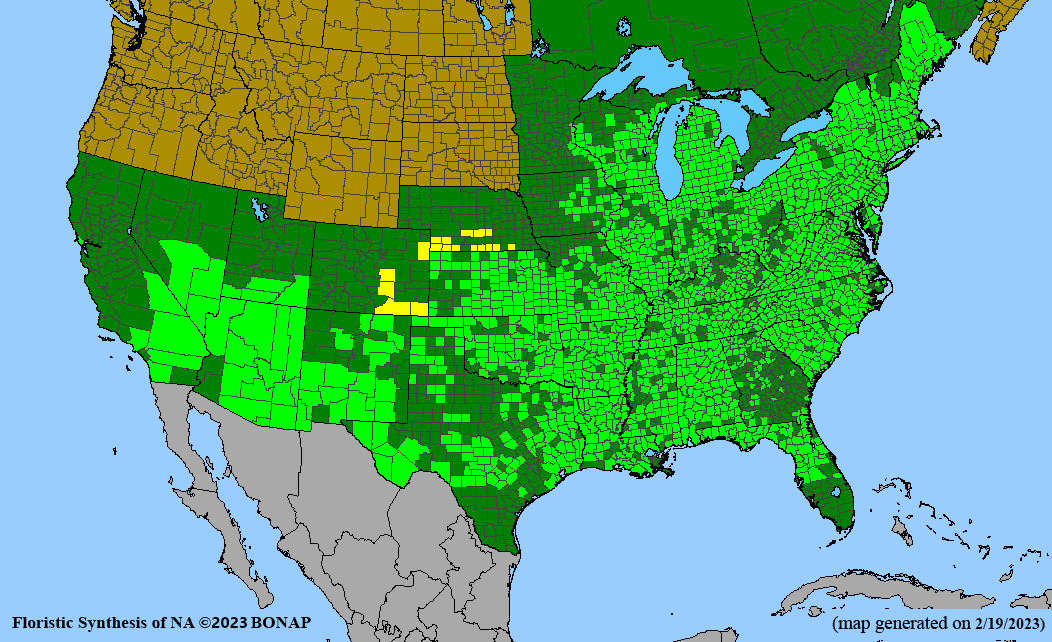
General Comments
The striking scarlet flowers of cardinalflower attract human admirers as well as hummingbirds, their main pollinators. The stamens and stigma of the flowers are held out from the floral tube at the right height and distance to paint the head of a hummingbird with pollen - or to receive pollen from another flower. Individual plants usually don’t live longer than two to three years in seed production or garden settings, but they produce numerous miniscule seeds so that the population can persist. This species is not difficult to propagate from seed, but it is a little tricky to time the harvest effectively, and the seed yield is on the low end, at least by weight.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: Cold/moist stratification for 60 days improves germination. Seeds also respond to gibberellic acid, but we have not found the optimal concentration to produce high rates of germination along with healthy, normal seedlings. Stratification is reliable and works well.
Sowing: Surface sow in the greenhouse 2-3 months before the last frost. Use caution when watering to avoid splashing seed. Seedlings are very tiny but fast growing, producing many fibrous roots and a leafy basal rosette, and starting the seeds in wide plugs that allow them room works well (e.g., 6X6 plug inserts in a standard 10X20 greenhouse flat).
Transplanting: When plugs are well rooted, move them outside to harden off and transplant them at 8-12 in spacing into irrigated rows with plastic mulch after danger of frost is past.
- Stand management
Weeds: Prepare a clean, weed-free bed and use plastic mulch to suppress weeds in the planting year. Widening the holes or removing the mulch in the second growing season allows new offsets to grow, producing small clumps of stems. Hand weed or rogue out small-seeded weeds to avoid contaminating the seed crop. We have interplanted cardinalflower with a cespitose, wetland sedge (Carex scoparia), and this seems to suppress weed growth and provides some support to the lobelia plants without reducing their seed yield compared with a previous, monospecific plot. A side benefit is that we obtained another seed crop, though the sedge had to be hand-harvested.
Pests: The larva of a specialist weevil (Cleopomiarus hispidulus) feeds on seeds in developing capsules and may affect seed yield in some years. We have reared weevils from affected capsules and noted many capsules with apparent exit holes from the emergence of adult weevils. These are native insects and part of the natural ecology of this plant species but may be problematic for seed production.
Diseases: None noted.
Hybridization risk: This species is known to hybridize with related species in the genus Lobelia such as L. siphilitica and L. spicata. Maintain separation distances between production fields of these species.
- Seed production
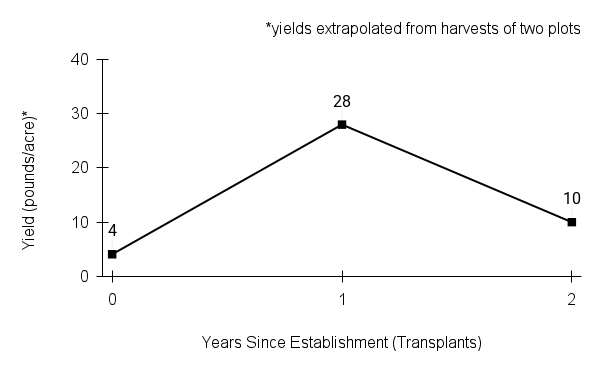 First harvest: There may be a small harvest in the planting year.
First harvest: There may be a small harvest in the planting year.Yield/acre: 4 to 30 pounds per acre (yields extrapolated from harvests of two plots at TPC)
Stand life: Plants are short-lived in seed production settings. Peak harvest occurs in the year after planting and declines in subsequent years apparently due to mortality of plants in the plot.
Flowering date: late July to early September (a few flowers may hang on longer)
Seed maturity/Harvest date: mid September through mid October
Seed retention: High risk of shattering. Once capsules open, seed is shaken out easily by any slight movement.
Harvest date range at TPC (2011-2023): September 11 - October 19
Recommended harvest method: Hand clip stems as capsules mature. This can be tricky to judge since the capsules are partially hidden within the persistent, greenish calyx and beneath remnants of the withered flower. They mature from the bottom up along a stem. By the time the last flowers bloom at the tip, generally at least half of the capsules are mature, and the lowest ones will have already opened. This is a good time to clip the stems and bring them into a sheltered place to ripen and dry. For a larger stand, combining when most plants have stopped flowering and about half of the capsules are mature on most stems should be effective, as it is for great blue lobelia, Lobelia siphilitica.
- Seed cleaning and storage
Cleaning process: Shake and/or crush dried stems to release seed from capsules. Pass hand-collected material through 1/4 in mesh to remove larger pieces of stems and leaves, then air-screen.
Seed storage: Cool/dry (33-50° F, 30-50% RH); a seedlot stored under these conditions for 8 years had 84% viability.
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone Iowa
Cultivated varieties (cultivars): Selections have been made for the horticultural trade.
- References
Belov, B., Bueche, B., Moisset, B. (2021, May 29) Species Cleopomiarius hispidulus. Bug Guide. https://bugguide.net/node/view/362089
Chayka, K. (n.d.). Lobelia cardinalis (cardinal flower). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/cardinal-flower
Hilty, J. (2019). Cardinal flower - Lobelia cardinalis. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/cardinal.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Mahr, S. (n.d.). Cardinal flower, Lobelia cardinalis. Wisconsin Horticulture Division of Extension. https://hort.extension.wisc.edu/articles/cardinal-flower-lobelia-cardinalis/
Missouri Botanical Garden. (n.d.). Lobelia cardinalis. Missouri Botanical Garden - Plant Finder https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=278870
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Prairie Moon Nursery. (n.d.). Lobelia cardinalis. https://www.prairiemoon.com/lobelia-cardinalis-cardinal-flower-prairie-moon-nursery.html
USDA NRCS National Plant Data Team. (n.d.). Lobelia cardinalis L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=LOCA2
Species Guide Updated 1/5/2026
closed bottle gentian
closed bottle gentian dickeye
Gentiana andrewsii, Griseb.
Alternate Common Names: bottle gentian, closed gentian, barrel gentian, blind gentian, cloistered gentian, Andrew’s gentian, fringe-top bottle gentian
Family: gentian family (Gentianaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from a stout taproot.
Height: 1-2 ft

- Leaves and stem

Leaves opposite, lance-shaped, 4 in long and up to 2 in wide, with a smooth margin, glossy and hairless surface, and sessile (stalkless) on the stem but not clasping; stem smooth, light green or purple, unbranched.
- Flower, fruit and seedhead
Flower: Five petals fused into a tube with an elongated, balloon-like shape that never opens, 1 to 1 1/2 in long, violet-blue (occasionally white or pink), in dense clusters at stem ends and upper leaf axils.
Fruit/seed head: Petals wither but are retained as a sheath around the developing capsule, 1 in long, straw-colored at maturity, with two persistent, curved styles at the tip that protrude from the dried corolla when ripe and a seam that splits along each side when seed matures, releasing numerous tiny, light, winged seeds that are dispersed by wind or water.
Pollination: This species is effectively pollinated only by bumble bees that are strong enough to push their way into the closed, bottle-shaped flowers.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 280,000 (IA NRCS)
1000 seed weight: 0.06g (Seed Information Database)
Description: Very tiny, teardrop-shaped golden seeds are surrounded by a flattened pale wing, making them look like miniature fried eggs; inclusive of the wing, they are about 1 mm wide and 2 mm long.
Typical seed test
PLS: 85% (n = 11)
Purity: 85% (n = 10)
Germination: 4% (n = 9)
Dormancy: 82% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist soil; partial to full sun; prairies, openings in floodplain forests, thickets, fens; Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest; irrigation is needed for seed production.
Conservation status: Global- G5, secure; Delaware and Massachusetts- S1, critically imperiled; Maryland and Vermont- S2, imperiled; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe)
General Comments
One of the most charming plant-insect relationships to observe in the prairie is that between closed bottle gentian and its sole pollinators: bumble bees. Even large, strong bumble bees have to work hard to force their way past the tightly pleated tips of the closed flowers. Smaller bumble bees disappear completely inside the “bottle” while the rear ends of larger bumbles protrude as they feed actively on the nectar inside. This species and other late flowering gentians are important resources for the bumble bee gynes (new females) that emerge in fall and must be well fed to survive the winter and start new colonies in the spring. Closed bottle gentian grows among sedges and grasses in wet prairies and at the edges of fens in our region. Irrigation is needed for seed production.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: 90 day cold/moist stratification or treat with 125 ppm to 250 ppm GA-3 solution for 24 hours just prior to sowing.
Sowing: Surface sow on saturated potting mix at least 4 months before last frost or expected planting date. Sow directly into plugs (aim for 3-5 seeds per cell) as seedlings are delicate and easily damaged by dibbling. Use caution when watering to avoid splashing seed from soil. Alternatively, seed may be started in phytoagar with 50-60 ppm GA-3 and transplanted to plugs with a small piece of agar left around the root. This method produces high rates of germination. Seeds may otherwise be slow and spotty in germination, and seedlings are always very small and slow growing. If seed in soil germinates poorly, spraying the soil surface with 500 ppm solution of GA-3 can help produce a new flush of seedlings. Be careful when spraying this solution to avoid contaminating surfaces or potting media, as many species are very sensitive to it.
Transplanting: Keep seedlings in plug flats until well-rooted, usually several months. Seedlings started in the greenhouse in spring will remain as rosettes until late summer to early fall, when some seedlings may begin to form leafy stems. At this point, they will still be fairly delicate plugs but should have sufficient root growth to be hand transplanted into irrigated, plasticulture production rows. If planted in fall, some plugs may frost-heave, but if re-seated in their holes in time, they may survive. Walk the rows frequently as the ground thaws, and seat plugs in their holes using a carefully placed boot.
- Stand management
Weeds: Prepare clean, weed-free beds. Plastic mulch suppresses weeds in the first season or two. We have tried planting closed bottle gentian with a wetland grass, bluejoint, to suppress weeds and provide some support and protection from deer. A better companion might be a tufted (cespitose) wetland sedge such as Carex scoparia, as bluejoint spreads by rhizomes to form dense stands that begin to suppress the gentians, except those on the edges of the row, in the third growing season.
Pests: Deer like to eat the flower clusters. Weeds or a companion grass within the plots discourage this. The larvae of a moth (Endothenia hebesana) feed on developing seeds within capsules.
Diseases: None noted.
Hybridization risk: This species is known to hybridize with other species in the genus Gentiana and a related genus (Gentianella quinquefolia). Maintain separation distances between plots of these species.
- Seed production
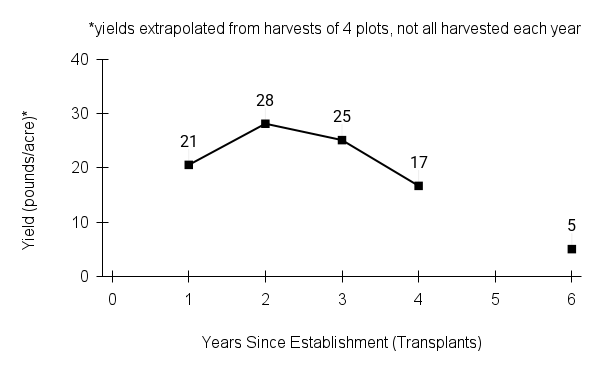 First harvest: Expect the first flowers and seed set in the year after planting. Peak yields are in the second through fourth growing seasons.
First harvest: Expect the first flowers and seed set in the year after planting. Peak yields are in the second through fourth growing seasons.Yield/acre: 20-30 pounds per acre of very small, light seed (extrapolated from harvests of four plots at TPC)
Stand life: Stands may persist for six years or more, but yields decline in the fifth year and later.
Flowering date: late August through mid October in northeast Iowa
Seed maturity/Harvest date: late October through early November in northeast Iowa
Seed retention: Fairly high risk of shattering once capsules extend past the dried corolla tube and split open, especially during high wind events. The seeds are very light and easily dispersed.
Harvest date range at TPC (2003-2006): September 9 - November 20
Recommended harvest method: Hand pick as the capsules ripen.
- Seed cleaning and storage
Cleaning process: Shake and/or crush hand collected material to release seeds from capsules, pass through 1/4 in and 1/8 in mesh to remove larger particles, then airscreen. Static buildup can be a problem in handling this seed.
Seed storage: Cool/dry (33-50° F, 30-50% RH); seed apparently lasts only a few years in storage.
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI (northern Iowa, aligned with the Generalized Provisional Seed Zones of the US Forest Service)
- References
Barnd, B., Hardy, R. Hatfield, M.J., O’Connor, M., Tony, Zimlich, R.L. (2025, February 8) Species Endothenia hebesana - Verbena Bud Moth - Hodges#2738. BugGuide. https://bugguide.net/node/view/132143
Bower, Andrew D.; St.Clair, J. Bradley; Erickson, Vicky. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Chayka, K. (n.d.). Gentiana andrewsii (bottle gentian). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/bottle-gentian
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Bottle gentian. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 203). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Bottle gentian - Gentiana andrewsii. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/bt_gentianx.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Closed gentian. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 258–259). University of Iowa Press.
USDA Forest Service and Southern Regional Extension Forestry. (n.d.). Propagation protocol database. RNGR - Reforestation, Nurseries, and Genetic Resources. https://npn.rngr.net/propagation
USDA NRCS National Plant Data Team. (n.d.). Gentiana andrewsii Griseb. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=GEAN
Species Guide Updated 12/23/2025
common boneset
common boneset sagem
Eupatorium perfoliatum L.
Alternate Common Names: boneset, thoroughwort
Scientific Synonyms: Eupatorium chapmanii Small, Eupatorium perfoliatum var. colpophilum Fernald & Griscom, Eupatorium perfoliatum var. cuneatum Engelmann
Family: aster or sunflower family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial, short-rhizomatous, spreads slowly to form small colonies.
Height: 2-4 ft

- Leaves and stem

Leaves join around the stem making them look like one leaf (perfoliate) and then taper to a point, opposite arrangement (rarely whorled), leaf margins are wavy with small teeth (crenulate), leaf surfaces have a wrinkled appearance, upper and lower leaf sides are hairy; stems are hairy, erect, and branched in the upper portion of the plant.
- Flower, fruit and seedhead
Flower: Tiny heads (1/4 in) of up to 15 disc florets (no visible “petals” or rays), grouped in flat-topped to slightly domed clusters of dozens to hundreds of heads; flower clusters appear fuzzy due to thin styles that extend from each floret.
Fruit/seedhead: Clusters become fluffy from the center outwards as seeds mature and pappus expands.
Pollination: Insects, particularly bees, butterflies, flies, beetles, and wasps.

- Seed
Seed characteristics
Seeds per ounce: 160,000 (IA NRCS)
1000 seed weight: 0.11 g (Seed Information Database)
Description: Long slender, dark gray seeds (achenes) up to 2.5 mm long with a short tuft of white hairs (pappus).
Typical seed test
PLS: 68% (n = 6)
Purity: 71% (n = 6)
Germination: 16% (n = 5)
Dormant: 81% (n = 5)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet, organic-rich soil; full sun; wet pastures, sedge meadows, fens; The USDA classifies it as an Obligate Wetland species in the Midwest region. It benefits from irrigation in production systems.
Conservation status: Global- G5, secure; Kansas- S3, vulnerable (NatureServe)

General Comments
The clouds of sweet-scented flowers attract a diverse assemblage of pollinating insects. Bitter compounds in the foliage deter mammalian herbivores, although some moth larvae use common boneset as a host plant. This species has traditional medicinal and ceremonial uses among Native tribes within its range, and was adopted as a treatment for colds and fevers by colonial settlers. It is currently under investigation by researchers in Germany and India for use in treatment of viral illnesses such as colds, flu, and dengue fever as well as malaria. Caution: this plant also contains phytochemicals that may be toxic to the liver.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience direct seeding this species for seed production. It reportedly has low germination rates in direct seedings. High seeding rates and fall planting are recommended.
Greenhouse
Seed pre-treatment: 60 days cold-moist stratification.
Sowing: Seed is small and should be surface sown. If started in germination flats, transplant to individual plugs when seedlings have their first pair of true leaves, about 4 weeks after seeding.
Transplanting: Seedlings are ready to transplant to the field about 6-8 weeks after being transferred to plugs, when their roots are well-branched and numerous root tips are visible at hole(s) in the base of the plug. Pop out a few plugs to check for adequate root development that will provide sturdy plugs for planting. Seedlings are fast growing and may need to be clipped back before transplanting to improve the shoot:root ratio. A week or two before transplanting, move flats outside to ‘harden off.’ (See Propagation chapter in General Information for more details).
- Stand management
Weeds: In the first season after transplanting, weeds are suppressed by a plastic weed barrier. Plants spread slowly by short rhizomes; in second and subsequent years, holes in plastic may need to be expanded or plastic removed to make room for new stems. Well-established plots shade out most weeds.
Pests: None noted.
Diseases: None noted.
Soil moisture: Irrigation is necessary in most soils to obtain maximum seed yield.
- Seed production
 First harvest: Plants flower and set a little seed the first year when transplanted in spring.
First harvest: Plants flower and set a little seed the first year when transplanted in spring.Yield: 80-130 pounds/acre (based on 1 plot)
Stand life: unknown
Flowering date: August - September in northeast Iowa
Seed maturity/Harvest date: mid-September - mid-October
Seed retention: Seeds are wind dispersed soon after maturity, when fluff (pappus) expands in late September through mid-October.
Harvest date range at TPC (2022-2023): Sept 15 - Oct 7
Recommended harvest method: Watch for the centers of seed clusters to begin shattering, and pick early maturing seed heads (clip stalks below seed clusters). If some heads in a cluster are still closed (not fluffy), pull apart a few heads to see if the seeds are dark colored and separate easily from the base (receptacle). Combine the rest of the plot at peak maturity. Turn off air or combine will disperse the fluffy seeds.
- Seed cleaning and storage
Cleaning process: If hand clipped, run dried material through a 1/4 in mesh to thresh seed from stalks. Use a brush machine (soft bristles, minimum vacuum) to remove pappus. Seed is fragile, and some seed is dehulled, even when soft bristles are used. Winnow with a box fan to separate seed from most of the pappus and chaff. Airscreen 2-3 times to finish cleaning. See Appendix C for specific settings.
Seed storage: cool/dry (33-50° F, 30-50% RH) for up to 3 years (USDA Plant Fact Sheet).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI
- References
Belt, S. (2009). Plant fact sheet for common boneset (Eupatorium perfoliatum L.). USDA-Natural Resources Conservation Service, Norman A. Berg National Plant Materials Center, Beltsville, MD 20705.
Chayka, K. (n.d.). Eupatorium perfoliatum (common boneset). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/common-boneset
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Boneset. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 79). University of Wisconsin-Madison Arboretum.
Eupatorium perfoliatum. (n.d.). Prairie Moon Nursery. https://www.prairiemoon.com/eupatorium-perfoliatum-boneset-prairie-moon-nursery.html
Hilty, J. (2019). Common boneset - Eupatorium perfoliatum. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/cm_boneset.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
SER, INSR, RBGK, Seed Information Database (SID). (2023). Eupatorium perfoliatum. https://ser-sid.org/species/e29e87df-3177-43f1-bfcd-bc052339de84
USDA NRCS National Plant Data Team. (n.d.). Eupatorium perfoliatum L. USDA plants database. https://plants.usda.gov/plant-profile/EUPE3
Siripun, K. C., & Schilling, E. E. (2020, November 6). Eupatorium perfoliatum Linnaeus. Flora of North America. http://floranorthamerica.org/Eupatorium_perfoliatum
Species Guide Updated 12/18/2024
common sneezeweed
common sneezeweed dickeye
Helenium autumnale, L.
Alternate Common Names: sneezeweed, sneezewort, swamp sunflower
Scientific Synonyms: Helenium autumnale var. canaliculatum (Lamarck) Torrey & A. Gray, Helenium autumnale var. fylesii B. Boivin, Helenium autumnale var. grandiflorum Torrey & A. Gray, Helenium autumnale var. montanum (Nuttall) Fernald, Helenium autumnale var. parviflorum (Nuttall) Fernald, Helenium latifolium Helenium parviflorum
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with shallow, fibrous rootstock, spreading slowly from rhizomes to form clumps.
Height: 2-5 ft

- Leaves and stem
Leaves alternate, lance-shaped, 3 to 6 in long and 1 in wide, stalkless, short-hairy, with small, irregularly spaced teeth on leaf edges and a central, white vein; stem is stiff, angular, and “winged” by extensions of the leaf bases that run down the stem.
- Flower, fruit and seedhead
Flower: Composite heads, 1-2 in wide, are nearly globular with numerous golden yellow disk florets and 10-15 bright yellow ray florets that are narrow at the base and 3-lobed at the tip; up to 100 heads are in a branching arrangement at the top of the plant.
Fruit/seed head: Disk of the flowerhead turns into a head of brown achenes that can persist into winter, although seeds shatter as plants rub against each other on windy days.
Pollination: Insects, primarily bees, but also beetles, flies, and wasps.

- Seed
Seed characteristics
Seed weight:
[Seeds per ounce: 130,000 (IA NRCS)
1000 seed weight: 0.53g (Seed Information Database)
Description: “Seed” is a grayish-tan, ridged achene about 1.5 mm long with short silvery hairs and a pappus of papery scales.
Typical seed test
PLS: 86% (n = 7)
Purity: 93% (n = 7)
Germination: 28% (n = 6)
Dormancy: 52% (n = 6)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soil; partial to full sun; moist prairies and meadows, fens, along streams, ponds and lakes, marshes, ditches; Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest; irrigation is recommended for seed production unless the site is reliably moist.
Conservation status: Global- G5, secure; Vermont- S1, critically imperiled; Kansas, Massachusetts, and Wyoming- S3, vulnerable; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
General Comments
Common sneezeweed blooms in late summer through October in our area, making it an important resource for late season pollinators. A population of these plants is a great place to look for diverse bumble bees in fall, including vulnerable species such as the Southern Plains Bumble Bee. The pollen of sneezeweed sticks to the hairs of the insects that visit it for nectar and does not blow in the wind or cause seasonal allergies in people. The common name sneezeweed refers to Indigenous medicinal practices involving the use of dried, powdered flowerheads as a snuff to induce sneezing in people suffering from congestion. This species is easy to establish and maintain for a few years in irrigated production plots, and harvest and seed cleaning are relatively uncomplicated. Sneezeweed has become quite popular in the horticulture industry for its late-season color, and there are a number of cultivated varieties.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Cold/moist stratification for 30-60 days may improve germination at ambient greenhouse temperatures in February to March.
Sowing: Surface sow in the greenhouse about 2-3 months before the last frost. Use caution when watering to avoid splashing seed from the soil. A heat mat may improve germination, especially if daytime greenhouse temperatures are below 75°F, and if seed was not stratified.
Transplanting: When seedlings have formed well-rooted plugs, move them outside to harden off, then transplant into irrigated plasticulture beds at 12 inch intervals, after danger of frost has passed.
- Stand management
Weeds: Prepare a clean, weed-free bed. Plastic mulch suppresses most weeds in the first year. Mow or cultivate between rows. Hand weed or rogue to prevent weed seed from contaminating the seed crop, paying special attention to small-seeded members of the aster family (e.g., frost aster, goldenrods). We have planted H. autumnale with bluejoint as a companion grass. While sneezeweed is probably not long-lived anyway, competition from bluejoint may have accelerated its decline. However, it also suppressed most weeds.
Pests: None noted.
Diseases: None noted.
- Seed production
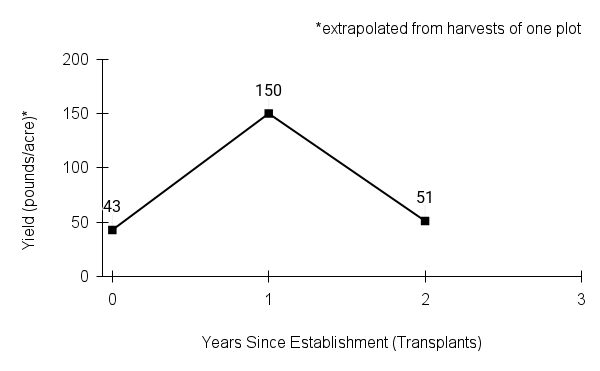
First harvest: Plants flower and set seed in the establishment year (from transplants).
Yield/acre: 40-150 pounds per acre, extrapolated from harvests of one plot at TPC.
Stand life: Peak yield appears to occur the year after planting (year 2) and subsequently decline, but this is based on experience with only one plot.
Flowering date: early August through October in northeast Iowa
Seed maturity/Harvest date: late September through mid October in northeast Iowa
Seed retention: Moderate risk of shattering; mature seedheads break up when rubbed against other plants in high winds.
Harvest date range at TPC (2023-2025): September 25 - October 17
Recommended harvest method: Hand pick early maturing plants, then combine at peak maturity of plot.
- Seed cleaning and storage
Cleaning process: Pass harvested material through 1/2 in and 1/4 in mesh to remove larger particles. Brush (soft bristles) to remove persistent dried corollas from achenes, then airscreen repeatedly.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI (northern Iowa), aligned with the Generalized Provisional Seed Zones of the US Forest Service
Cultivated varieties (cultivars): A number of selections have been made for the horticultural trade.
- References
Bierner, M. W. (2020, November 5). Helenium autumnale Linnaeus. Flora of North America. http://floranorthamerica.org/Helenium_autumnale
Chayka, K. (n.d.). Helenium autumnale (sneezeweed). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/sneezeweed
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Common sneezeweed. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 82). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Common sneezeweed - Helenium autumnale. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/sneezeweed.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
USDA NRCS National Plant Data Team. (n.d.). Helenium autumnale L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=HEAU
Species Guide Updated 12/30/2025
compass plant
compass plant dickeye
Silphium laciniatum L.
Alternate Common Names: compass plant, rosinweed, turpentine plant, polar plant, pilot-weed
Scientific Synonym: Silphium laciniatum var. robinsonii L. M. Perry
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial, large 15 ft central taproot.
Height: 3-10 ft

- Leaves and stem

Leaves alternate, 12 - 24 in long, deeply lobed, densely covered in coarse hairs; stems are rounded in cross section and covered in long, coarse, spreading hairs, somewhat woody late in the season.
- Flower, fruit and seedhead
Flower: Yellow composite flower heads, 3-4 in diameter, several per flowering stem.
Fruit/seedhead: Seeds (technically achenes) form only from outer fertile flowers of the central disk.
Pollination: Primarily bees

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 660 (IA NRCS)
1000 seed weight: 78.05 g (Seed Information Database)
Description: ‘Seeds’ are flat fruits (achenes), 3/8 – 1/2 in long, with a broad wing around margins, making it difficult to get a good separation between filled and unfilled seed. No plume.
Typical seed test
PLS: 85% (n = 11)
Purity: 88% (n = 11)
Germination: 14% (n = 9)
Dormant: 73% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Wet-mesic to dry-mesic soil conditions; full sun; typically on high quality prairie remnants, railroads, roadsides, glades, savannas; preference is for moist, well-drained soils for seed production.
Conservation status: Global- G5, secure; Colorado- SH, possibly extirpated; Ohio, Oklahoma, and Virginia- S1, critically imperiled; Michigan- S1/S2, critically imperiled to imperiled; Kentucky and Tennessee- S2, imperiled; South Dakota- S3, vulnerable (NatureServe)

General Comments
Compass plant is a long-lived, tap-rooted species. An individual seedling may take 2-4 years to flower in production, and flower every year thereafter for a few years. In a prairie, an individual may take 5-10 years to flower, and subsequently flower every other year or so. Silphium species (congeners) will hybridize with one another in nature, so they should be properly isolated from related species for seed production purposes (i.e. cupplant, Silphium perfoliatum; rosinweed, Silphium integrifolium; prairie dock, Silphium terebinthinaceum; etc.) (Fisher 1966, Clevinger and Panero 2000)
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species
Greenhouse
Seed pre-treatment: Wet stratify 8-12 weeks at 40° F.
Sowing: Sow seed in greenhouse two months before last frost free date.
Transplanting: Transplant into bare soil in rows or weed barrier at 12 in intervals after all danger of frost is past.
- Stand management
Weeds: Post emergence grass herbicide, tillage, hand roguing. Plastic mulch is not recommended for this species. In an informal trial at TPC, seedlings grown in plastic mulch grew more slowly and took longer to reach maturity than seedlings grown in a biodegradable paper mulch.
Pests: None noted.
Diseases: None noted.
Hybridization risk: This species is known to hybridize with related species Silphium integrifolium, S. perfoliatum, and S. terebinthinaceum. Plan plot layouts to maximize separation among these species.
- Seed production
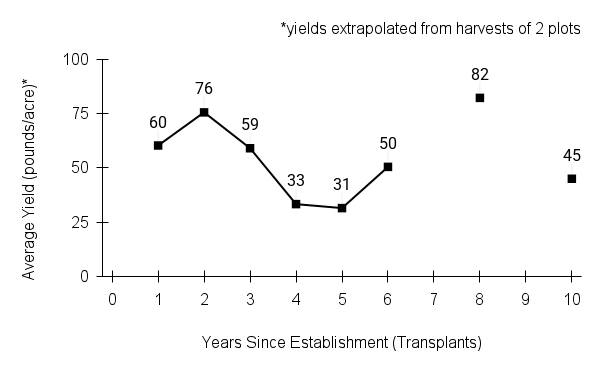 First harvest: Some flowering and seed set end of second year, most will flower during the third growing season from greenhouse grown transplants.
First harvest: Some flowering and seed set end of second year, most will flower during the third growing season from greenhouse grown transplants.Yield: 40-130 bulk pounds/acre
Stand life: Peak harvests third year. Plants are very long-lived, but seed production may decline significantly in the fourth to fifth year after planting. Harvests from TPC plots also provide some evidence for fluctuating yields over time.
Flowering date: Early July to mid-August.
Seed maturity/Harvest date: Mid-August to mid-September.
Seed retention: Shattering occurs as seeds mature and dry, end of August into September.
Harvest date range at TPC (2003-2012): Aug 22 - Oct 14
Recommended harvest method: Hand collect as seed ripens for most efficient harvest of small plots, combine for larger stands when seed is mostly mature and before significant shattering.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping through 1/2 in mesh and sifting through 1/4 in mesh (most seed will be retained on top of 1/4 in mesh). Brushing with soft bristles helps to break up inert matter to achieve better separation. Air-screen repeatedly to clean.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zone 1 (northern Iowa), Zone 2 (central Iowa), and Zone 3 (southern Iowa)
- References
Chayka, K. (n.d.). Silphium laciniatum (compass plant). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/compass-plant
Clevinger, J. A. (2020, November 5). Silphium laciniatum Linnaeus. Flora of North America. http://floranorthamerica.org/Silphium_laciniatum
Hilty, J. (2019). Compass plant - Silphium laciniatum. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/compassx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 50–51). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Compass plant. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 180–181). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Silphium laciniatum L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SILA3
Species Guide Updated 12/10/2025
fourflower yellow loosestrife
fourflower yellow loosestrife dickeye
Lysimachia quadriflora Sims
Alternate Common Names: four-flower yellow loosestrife, linear-leaf loosestrife, prairie loosestrife
Scientific Synonyms: Lysimachia longifolia Pursh; L. revoluta Nuttall; Nummularia longifolia (Pursh) Kuntze; Steironema longifolium (Pursh) Rafinesque; S. quadriflorum (Sims) Hitchcock
Family: myrsine family (Myrsinaceae), formerly classified in the primrose family (Primulaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with sprawling stems that are often supported by surrounding vegetation, spreading slowly from short rhizomes to form clumps of several stems.
Height: 1-2.5 ft

- Leaves and stem
Leaves very narrow (linear), 1 1/4 to 3 1/2 in long and only up to 1/4 in wide, opposite and often with smaller secondary leaves at nodes making them appear whorled, leaves glossy and mostly hairless, often turning red in fall; stems smooth and unbranched or branching in the top half of the plant.
- Flower, fruit and seedhead
Flower: Flowers regular, 1-in wide, 5-parted, nodding on slender petioles, emerging singly or in loose clusters of up to 4 flowers from upper leaf axils; petals bright yellow, often with ragged edges and faint reddish streaks or spots.
Fruit/seed head: Fruit is a nearly spherical, glossy capsule with a persistent point (the style), opens from the top at maturity and seed scatters out as the plant is shaken by the wind or passing animals.
Pollination: Insects, including generalist and specialist bees.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 90,000 (IA NRCS)
1000 seed weight: 0.28 g (Seed Information Database)
Description: Seeds 1-1.5 mm long, 3-sided, irregular in outline, concave on one side, dark or reddish brown, with a rough surface texture. Seed image includes ruler with mm markings.
Typical seed test
PLS: 90% (n=3)
Purity: 93% (n=3)
Germination: 0.33% (n=3)
Dormant: 97% (n=3)
(averages obtained from 3 tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soils in full sun; wet prairies, fens, seeps, ditches; Wetland Indicator Status is OBL (obligate, almost always occurring in wetlands) in the Midwest; irrigation is needed for seed production.
Conservation status: Global- G5 (secure); Kentucky, West Virginia- SH (possibly extirpated); Alabama, Georgia- S3 (vulnerable); South Dakota, Oklahoma, Tennessee, New York, Virginia- S1 (critically imperiled); in all other states in its native range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe)
[BONAP Map]
General Comments
If we want planted prairies to better resemble remnant prairies in their diversity and species composition, fourflower yellow loosestrife is a species that should be considered for wet soils. It can be found in most wet prairies, sedge meadows, seeps, and fens in our area and even in some wet ditches where native vegetation has persisted. This species is inconspicuous due to its size except when in flower or when the foliage turns red in the fall. It can be easily distinguished from other members of its genus in our region by its very thin (linear) leaves that are only up to about 1/4 in wide. The relatively weak, slender stems of fourflower yellow loosestrife seem to lean on taller grasses, sedges, and forbs for support, and some of the plants may sprawl a bit in production settings. The nodding, intensely yellow flowers produce oils rather than nectar and are visited by specialist bees that use the oils, mixed with pollen, to provision their larvae, as well as by generalist bees that use pollen.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: 90 days cold/moist stratification
Sowing: Surface sow in greenhouse 3 months before last frost free date. Use caution in watering to avoid splashing small seeds from soil. Seeds may be slow and irregular in germination, but keeping germination flats saturated (in a tray of standing water) may improve germination.
Transplanting: When plugs are well rooted, place them outside to harden off, then transplant at 8-12 in spacing in irrigated rows with plastic mulch.
- Stand management
Weeds: Prepare clean, weed free beds. Use plastic mulch to suppress weeds in the first year or two. Mow or cultivate between rows. Hand weed or rogue out small seeded weeds or large, competitive weeds.
Pests: None noted.
Diseases: None noted.
- Seed production
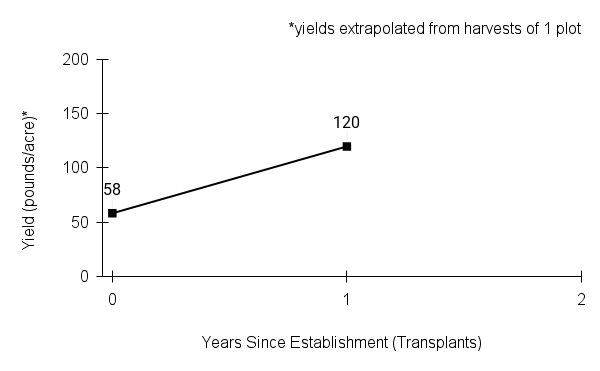 First Harvest: Some flowering and seed set in the first growing season from transplants. Yield doubled the following year.
First Harvest: Some flowering and seed set in the first growing season from transplants. Yield doubled the following year.Yield/Acre: 60-120 pounds per acre (extrapolated from harvests of one plot)
Stand Life: Unknown at this time, but productive stand life is probably 3-5 years. Invasion of the plots by weed species that benefit from irrigation may be a primary cause of decline.
Flowering Date: mid June through August in northeast Iowa
Seed Maturity/Harvest Date: late September to mid October
Seed retention: Moderate risk of shattering though some seed is retained in capsules into late October.
Harvest date range at TPC (2024-2025): Sept 26 - Oct 14
Recommended Harvest Method: Combine and hand. Uneven ripening in the field presents a challenge. Observe plot frequently when the plants begin turning red in the fall and watch for open capsules. Consider harvesting when about 10-20% of the capsules are open. Crush some of the closed capsules and observe for dark colored (maturing) seed. Hand harvesting early individuals is recommended, and stems with capsules that are immature and pass through the combine can be collected and allowed to dry, and this later maturing seed can be threshed with a stationary combine. These methods can help retain diversity in the timing of flowering and fruiting in the restoration seed supply.
- Seed cleaning and storage
Cleaning process: Pass combined material through 1/2 in and 1/4 in screens, then airscreen repeatedly.
Seed storage: Cool/dry (33-50° F, 30-50% RH); long term viability in storage unknown at this time.
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI (aligned with the Generalized Provisional Seed Zones of the US Forest Service)
- References
Bower, Andrew D.; St.Clair, J. Bradley; Erickson, Vicky. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Cholewa, A.F., Pipoly J.J. III, Ricketson, J.M. (n.d.). Myrsinaceae R. Brown. Flora of North America. http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=10598
Chayka, K. (n.d.). Lysimachia quadriflora (Prairie Loosestrife). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/prairie-loosestrife
Hilty, J. (2019). Prairie Loosestrife, Lysimachia quadriflora. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/pr_loosestrife.html
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: December 18, 2025).
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
USDA-NRCS. (2024). Native seeding calculator 2024 [Excel File]. Retrieved from https://efotg.sc.egov.usda.gov/#/state/IA/documents/section=4&folder=-6
USDA NRCS National Plant Data Team. (n.d.). Lysimachia quadriflora Sims, fourflower yellow loosestrife. USDA plants database. https://plants.usda.gov/plant-profile/LYQU
Species Guide Updated 12/19/2025
fringeleaf wild petunia
fringeleaf wild petunia dickeye
Ruellia humilis, Nutt.
Alternate Common Names: wild petunia, hairy petunia, hairy wild petunia
Scientific Synonyms: Ruellia ciliosa Pursh var. longiflora A. Gray, Ruellia humilis Nutt. var. calvescens Fernald, Ruellia humilis Nutt. var. depauperata Tharp & F.A. Barkley, Ruellia humilis Nutt. var. expansa Fernald, Ruellia humilis Nutt. var. frondosa Fernald, Ruellia humilis Nutt. var. longiflora (A. Gray) Fernald
Family: acanthus family (Acanthaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from a fibrous root system, often with multiple stems arising from the ground and sprawling outward, with a leafy, bushy appearance.
Height: 1-2 ft

- Leaves and stem
Leaves are opposite, lance- to oval-shaped, somewhat tapered, without teeth or lobes, and covered in coarse, spreading, whitish hairs; the hairy stems are somewhat sprawling.
- Flower, fruit and seedhead
Flower: The pale purple flower is made up of 5 corolla lobes fused into a funnel shape up to 3 in long, similar in form to a cultivated petunia; the calyx with five long, very slender, hairy lobes persists as the fruit ripens.
Fruit/seed head: Fruit is a thick-walled, tan to brown, dry, oval, two-chambered capsule less than 1 in long, with a sharp, curved “beak;” seeds are ejected ballistically at maturity.
Pollination: Insects, primarily long-tongued bees.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 5,200 (IA NRCS)
1000 seed weight: 5.15g (Seed Information Database)
Description: Seed is round and flattened like a lentil, dark brown, covered in hairs that expand when the seed is wetted, helping to maintain contact with the soil.
Typical seed test
PLS: 97% (n = 6)
Purity: 100% (n = 6)
Germination: 7% (n = 5)
Dormancy: 91% (n = 5)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to moist soil; partial to full sun; prairies, bluffs, woodland openings, along railroads, roadsides; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; full sun and well-drained mesic soils are recommended for seed production.
Conservation status: Global- G5, secure; Maryland, Michigan, North Carolina, Pennsylvania, South Carolina, and West Virginia- S1, critically imperiled; Wisconsin- S2, imperiled; Georgia, Minnesota, and Virginia- S3, vulnerable; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
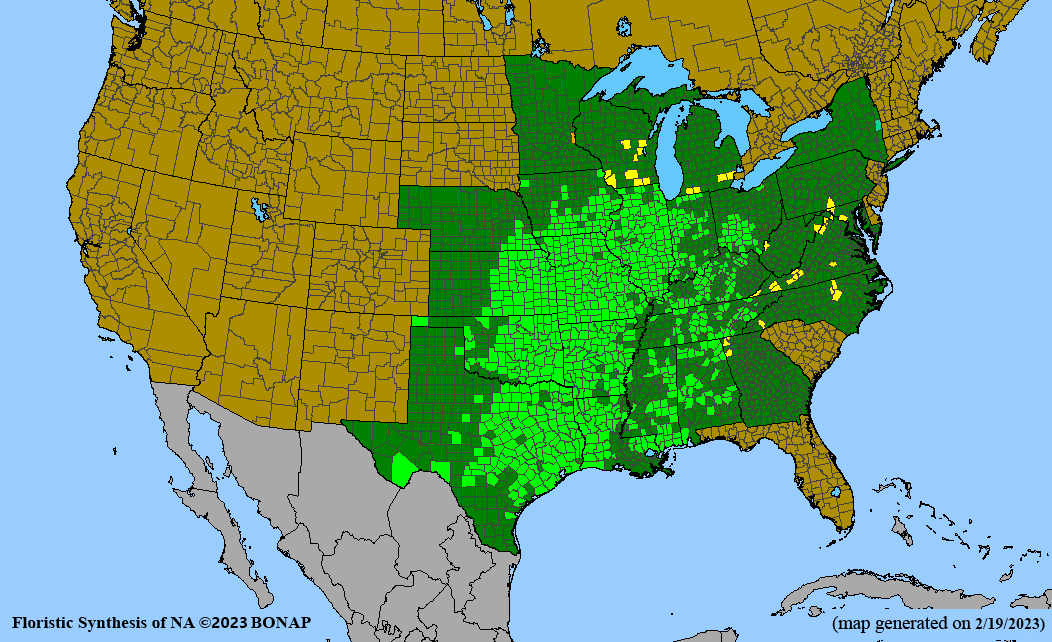
General Comments
Fringeleaf wild petunia flowers resemble the familiar garden petunia, but this species is in a different family, the Acanthaceae, which is uncommon in temperate zones. Each wild petunia flower fades and drops after a day, but the plants bloom generously from mid summer through fall. Wild petunias are small in stature and mostly encountered in prairies with a more open structure such as hill prairies. However, we have also stumbled across Ruellia in dense, mesic prairies, where such small plants might not be expected to survive the intense competition from taller grasses and forbs. Wild petunias are tolerant of a wide range of soil conditions and do well in mesic soils in seed production settings. The explosive seed capsules must be harvested before the seed is ejected. For a little fun, place some stalks with nearly mature capsules in a paper sack and give them to a friend. The sound of exploding seeds can be quite a surprise!
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Germination improves with 70 to 84-day (10 to 12 week) cold/moist stratification. We have also had some success using a 48-hour soak in 250 ppm GA-3, but the seedlings appeared less robust than those germinated after stratification. A higher concentration of GA-3 might produce better results.
Sowing: Sow seed, lightly covered with seed starting mix, in the greenhouse about 2-3 months before the last frost. If temperatures in the greenhouse are lower than 75°F during the day, a heat mat may improve germination.
Transplanting: When plugs are well-rooted, move the flats outside to harden off, then transplant at 8-12 in intervals into beds prepared with a weed barrier such as plastic mulch.
- Stand management
Weeds: Prepare, clean, weed-free beds prior to transplanting. Use plastic mulch or a weed barrier to reduce competition from weeds during the first year or two. Mow or cultivate between rows.
Pests: None noted.
Diseases: None noted.
- Seed production
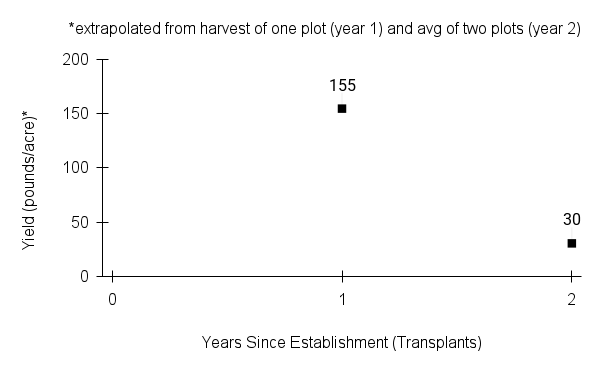 First harvest: Expect the first harvest in the year after transplanting.
First harvest: Expect the first harvest in the year after transplanting.Yield/acre: 30 - 150 pounds per acre (extrapolated from harvests of two plots)
Stand life: Peak yield in the second year (the year after transplanting). Our plots were retired after a disappointing harvest in year 3, so longer term yields are not known, but numerous plants have persisted (and perhaps reseeded themselves) in one of these former plot areas 10 years after the plot was abandoned.
Flowering date: early July through September in northeast Iowa
Seed maturity/Harvest date: mid to late October in northeast Iowa
Seed retention: Moderate risk of shattering. Capsules ripen rather slowly, but once mature, they eject seed explosively. Check plots frequently during ripening and observe for senescence of leaves and a change in color of capsules from green to tan or light brown.
Harvest date range at TPC (2015-2016): October 19 - 20
Recommended harvest method: Clip entire stalks when most stalks have some tan to brown capsules and capsules of some stalks have begun ejecting seeds. Dry the cut stalks in cloth bags or a bin with a screen over the top to prevent seed from shooting out.
- Seed cleaning and storage
Cleaning process: Pass the dried material through a coarse screen (1/2 in mesh) to remove stalks. If many unbroken capsules remain, brush with stiff bristles. Indent before or after air-screening.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 2 (central Iowa) and Zone 3 (southern Iowa)
- References
Baskin, J.M. & Baskin, C.C. (1982) Temperature Realtions of Seed Germination in Ruellia humilis and Ecological Implications. Castanea, 45(2)119-131 https://www.jstor.org/stable/4032944
Chayka, K. (n.d.). Ruellia humilis (Wild Petunia). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/wild-petunia
Hilty, J. (2019). Hairy wild petunia - Ruellia humilis. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/hw_petuniax.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Wild petunia. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., p. 141). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Ruellia humilis Nutt. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=RUHU
Species Guide Updated 12/29/2025
great blue lobelia
great blue lobelia dickeye
Lobelia siphilitica, L.
Alternate Common Names: blue lobelia, blue cardinal flower
Family: bellflower family (Campanulaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Short-lived perennial that produces small clumps of stems from vegetative offshoots.
Height: 1-4 ft

- Leaves and stem
Leaves alternate, elliptical to lanceolate, 2-6 in long, with margins that are serrated and wavy, and bearing scattered short hairs; stem is usually unbranched, ridged, with short hairs on the ridges.
- Flower, fruit and seedhead
Flower: Dense, spike-like racemes 6 to 24 inches long at ends of stems; flowers (1 - 1 1/2 in wide) are intensely blue (occasionally white or light blue), two-lipped with a nectar tube.
Fruit/seed head: Two-chambered capsule releases numerous tiny seeds from openings at the top.
Pollination: Insects, primarily bumble bees and other large-bodied bees including the federally endangered rusty patched bumble bee; butterflies may also visit the flowers.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 500,000 seeds/oz (IA NRCS)
1000 seed weight: 0.04g (Seed Information Database)
Description: Seeds are translucent, honey-colored, and elliptical in outline with an intricate, bumpy surface texture.
Typical seed test
PLS: 91% (n = 11)
Purity: 95% (n = 11)
Germination: 20% (n = 7)
Dormancy: 76% (n = 7)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soils; part shade to full sun; moist to wet prairies, fens, seeps, ditches, moist fields, openings in floodplain forests, shorelines; Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest; planting in full sun in reliably moist soil or under irrigation is recommended for seed production.
Conservation status: Global- G5, secure; Maine- SX, presumed extirpated; Massachusetts and Vermont- S1, critically imperiled; Wyoming- S1/S2, critically imperiled to imperiled; Louisiana and Mississippi- S3, vulnerable; secure (S5), apparently secure (S4), or unranked in other states within its range (NatureServe)
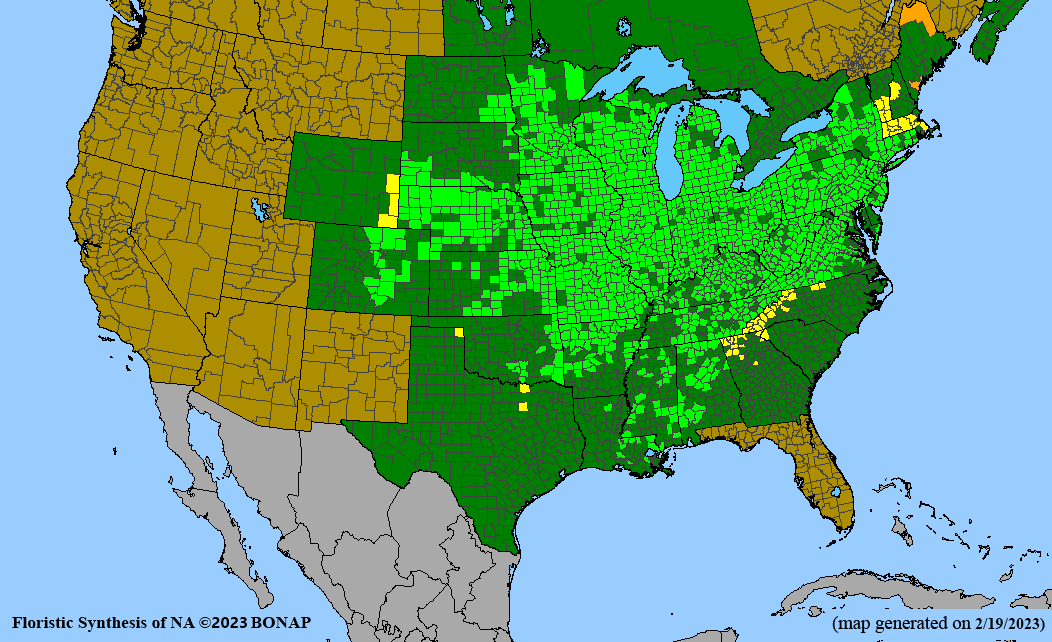
General Comments
Great blue lobelia blooms from mid summer through fall in prairie wetlands and woodland openings, providing nectar and pollen for its primary pollinators, long-tongued bumble bees. Hummingbirds and large butterflies also visit the flowers for nectar. The plant contains toxic and bitter compounds that make it unpalatable for most mammalian herbivores. Those same toxic alkaloids have a long history of use, in controlled doses, as medicines. The species name “siphilitica” alludes to Indigenous medicinal uses, including as a treatment for syphilis and other ailments, and one of the compounds found in great blue lobelia, lobeline, has been under recent investigation for treatment of addiction and depression. The brilliant blue color and long blooming season make these flowers attractive for home gardens as well as for larger plantings. Great blue lobelia plants are relatively short-lived in seed production, but they produce heavily for a couple of years. The seed is tiny but not particularly difficult to harvest or clean.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Cold/moist stratification for 60 days is recommended. Some sources suggest this step is unnecessary, however seed test results indicate that nearly 80% of seed has dormancy. Skipping the stratification step could reduce the proportion of plants with this trait in the restoration seed supply.
Sowing: Surface sow in the greenhouse at least 2 months before the last frost. Use caution when watering to avoid splashing seed. Seedlings are very tiny but fast growing, producing many fibrous roots and a leafy basal rosette, and starting the seeds in wide plugs that allow them room works well (e.g., 6X6 plug inserts in a standard 10X20 greenhouse flat).
Transplanting: When plugs are well rooted, move them outside to harden off and transplant them at 8-12 in spacing into irrigated rows with plastic mulch after danger of frost is past.
- Stand management
Weeds: Prepare a clean, weed-free bed and use plastic mulch to suppress weeds in the planting year. Widening the holes or removing the mulch in the second growing season allows new offsets to grow, producing small clumps of stems. Hand weed or rogue out very small-seeded weeds to avoid contaminating the seed crop.
Pests: None noted.
Diseases: None noted.
Hybridization risk: This species is known to hybridize with related species in the genus Lobelia such as L. cardinalis and L. spicata. Maintain separation distances between production fields of these species.
- Seed production
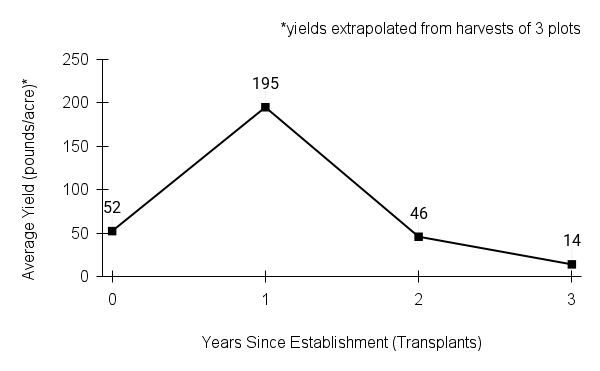 First harvest: Some flowering and seed set is expected in the establishment year, but peak harvest is in year 2.
First harvest: Some flowering and seed set is expected in the establishment year, but peak harvest is in year 2.Yield/acre: 50 - 200 pounds per acre (extrapolated from harvests of 3 production plots)
Stand life: Short-lived plants produce peak harvest in year 2, then production declines rapidly.
Flowering date: late July through September in northeast Iowa
Seed maturity/Harvest date: early to mid October in northeast Iowa
Harvest date range at TPC (2003-2011): Sept 5 - Oct 18
Recommended harvest method: Combine with air turned all the way down to avoid dispersing the tiny seeds. Hand pick early maturing stems to avoid losing their genetics from the production population.
- Seed cleaning and storage
Cleaning process: Pass combined material through 1/4 in mesh to remove larger debris, then airscreen. Crush handpicked stems, screen, then airscreen.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern Iowa), Zone 2 (central Iowa), Zone 3 (southern Iowa)
Cultivated varieties (cultivars): Horticultural selections are available for landscape design applications. However, the straight species by itself is a wonderful addition to a garden, is easy to grow from seed, and persists for years through self-seeding.
- References
Chayka, K. (n.d.). Lobelia siphilitica (blue lobelia). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/blue-lobelia
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Great blue lobelia. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 234). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Great Blue Lobelia - Lobelia siphilitica. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/gb_lobeliax.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Moerman, D. (2003). Native American ethnobotany database. BRIT. http://naeb.brit.org/
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Prairie Moon Nursery. (n.d.). Lobelia siphilitica. https://www.prairiemoon.com/lobelia-siphilitica-great-blue-lobelia-prairie-moon-nursery.html
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
USDA NRCS National Plant Data Team. (n.d.). Lobelia siphilitica L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=LOSI
Species Guide Updated 12/22/2025
longbract spiderwort
longbract spiderwort dickeye
Tradescantia bracteata, Small
Alternate Common Names: spiderwort, prairie spiderwort, bracted spiderwort, long-bracted spiderwort, sticky spiderwort
Family: dayflower and spiderwort family (Commelinaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with fibrous roots, spreading by rhizomes to form colonies.
Height: 1/2-1 1/2 ft
- Leaves and stem

Leaves 4 to 10 in long, floppy, creased at the midvein, with smooth surface (though often with hairs on margins) and parallel veins; base of leaves sheath the stem; stem is smooth, rarely branched; plants form clumps of multiple stems from the base.
- Flower, fruit and seedhead
Flower: Flowers in a terminal cluster of few to many flowers (occasionally also in leaf axils) with a prominent pair of bracts at their base as long as the stem leaves; flowers 1 in wide, 3-parted, violet to pink (occasionally white), bearing 6 stamens with hairy filaments and a single blue style; sepals are hairy, distinguishing T. bracteata from T. ohiensis (bluejacket or Ohio spiderwort); each flower blooms for one day (generally fading after morning), but flowering is staggered within a cluster; the hairy stalk supporting each flower stands up when flowering but droops once blooming is done.
Fruit/seed head: Seed capsules are 3-celled and 1/4 in long, obovoid shape, developing within the persistent hairy sepals; splitting open at maturity to release 2-6 seeds.
Pollination: Insects, primarily bees and flower flies (syrphids).

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 10,000 seeds/oz (IA NRCS)
1000 seed weight: 3.12g (Seed Information Database)
Description: Seeds dark gray (sometimes tan), oblong in outline with a dimple on one side and complex, grooved surface texture; 2-3 mm long and 1.5 mm wide.
Typical seed test
PLS: 88% (n = 11)
Purity: 95% (n = 11)
Germination: 7% (n = 10)
Dormancy: 85% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic soil; full sun; sand prairies, black soil prairies, woodland edges, along railroads, roadsides; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; well-drained loamy soils are recommended for seed production.
Conservation status: Global- G5, secure; Michigan- SX, presumed extirpated; Wyoming- S1, critically imperiled; Arkansas and Illinois- S2, imperiled; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked. (NatureServe)

General Comments
This is an attractive, early flowering species that persists through vegetative spread and reseeding in both high quality prairies and in somewhat disturbed areas. The flowers produce pollen but no nectar, hence they are visited primarily by bees that use pollen to provision their larvae or by syrphid flies that feed directly on pollen. Once the seed matures, the above ground parts of the plants wither and plants remain dormant until the next growing season, although small shoots may emerge in fall. This species is distinguished from similar species in our region by having glandular hairs on both sepals and flower stalks and prominent bracts under the inflorescences that are as long and wide as the stem leaves. Bracted spiderwort is generally a smaller plant than T. ohiensis (bluejacket or Ohio spiderwort) and can spread vegetatively to form large colonies, while bluejacket is more clumped.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: At least 90 days cold stratification.
Sowing: Sow about 1/4 inch deep in the greenhouse about 2 months before the last frost date.
Transplanting: When plugs are well rooted, take them outside to harden off for a week or two, then transplant in rows suitable for cultivation or at 8-12 inch intervals in plastic mulch.
- Stand management
Weeds: Prepare a clean, weed-free bed. Plastic mulch suppresses weeds in the establishment year but should be removed in subsequent years to allow vegetative spread. Mow or cultivate between rows. This species appears to be fairly intolerant of competition from weeds. Planting in rows between sheets of landscape fabric might facilitate harvest by allowing capture of seed that drops as it matures, but we have not tried this.
Pests: None noted.
Diseases: None noted.
Hybridization risk: This species is known to hybridize with other members of the genus Tradescantia such as T. ohiensis (bluejacket or Ohio spiderwort). Maintain separation distances between plots of these species.
- Seed production
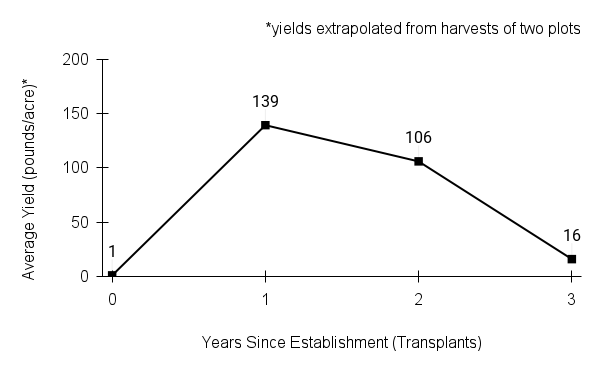 First harvest: Expect very little flowering and seed set in the first year. Peak yields occur in year 2 and/or 3.
First harvest: Expect very little flowering and seed set in the first year. Peak yields occur in year 2 and/or 3.Yield/acre: 100-140 pounds per acre in year 2 to year 3 (extrapolated from harvests of two plots at TPC)
Stand life: Productive stand life is 3-4 years. Yields begin to decline in year 3 or 4 and after.
Flowering date: mid May to mid June in northeast Iowa
Seed maturity/Harvest date: late June to early July in northeast Iowa
Seed retention: High risk of shattering. Seed drops from the plant as each capsule matures. There is no perfect way to time the harvest. Observe plants frequently as typical harvest dates approach. Turn over seedheads and look for dry, brown, open capsules among the hairy sepals. Plan to harvest when there are some mature capsules on most plants. If whole stalks are collected, capsules will continue to mature and release seed as they dry. If planted in rows between sheets of landscape fabric, it’s possible that dropped seed could be swept or vacuumed from the fabric.
Harvest date range at TPC (2003-2022): June 24 - July 22
Recommended harvest method: When most plants have some mature capsules in their seed heads, cut entire stalks and lay on tarp to dry. Provide ventilation and turn thick piles regularly to avoid mold growth. Seed will be released onto tarp as capsules mature and can then be easily screened from the dry stalks.
- Seed cleaning and storage
Cleaning process: Pass material through 1/2 inch mesh to remove dried stalks, then airscreen.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 2 (central Iowa)
- References
Chayka, K. (n.d.). Tradescantia bracteata (long-bracted spiderwort). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/long-bracted-spiderwort
Hilty, J. (2019). Prairie spiderwort - Tradescantia bracteata. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/pr_spiderwort.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Tradescantia bracteata Small. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=TRBR
Species Guide Updated 12/22/2025
meadow zizia
meadow zizia dickeye
Zizia aptera, (A. Gray) Fernald
Alternate Common Names: heart-leaved golden alexanders, heartleaf golden alexanders, heart-leaved alexanders, heart-leaved meadow parsnip, meadow parsnip, zizia
Scientific Synonyms: Zizia aptera (A. Gray) Fernald var. occidentalis Fernald, Zizia cordata W.D.J. Koch ex DC
Family: carrot and parsley family (Apiaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with a central taproot.
Height: 1-3 ft

- Leaves and stem

Basal and lower leaves are heart shaped (upper leaves 3-lobed) with long petioles, finely toothed margins, and a purple spot where the blade meets the petiole; leaves dark green, smooth and glossy; stems smooth, round, branched.
- Flower, fruit and seedhead
Flower: Flowers yellow, 1/8 in wide, with 5 petals that fold inwards; in compound umbels 1 1/2 to 3 in wide, each containing 7 to 15 small umbels with around 10-20 flowers each.
Fruit/seed head: Fruit is dark reddish brown with lighter brown ribs (not winged) and splits into two parts, each one-seeded.
Pollination: Insects, particularly small bees, flies, and beetles (including weevils).

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 12,000 (IA NRCS)
1000 seed weight: 1.53g (Seed Information Database)
Description: “Seed” (botanically a fruit) is oblong, 3mm long, dark brown, 5-ribbed.
Typical seed test
PLS: 94% (n = 10)
Purity: 100% (n = 10)
Germination: 4% (n = 9)
Dormancy: 73% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to moist soil; partial to full sun; prairies, bluffs, roadsides, woodland openings, shorelines, abandoned fields; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; well-drained loamy soils are recommended for seed production.
Conservation status: Global- G5, secure; Rhode Island- SH, possibly extirpated; Connecticut, Delaware, Nevada, and Oregon- S1, critically imperiled; Michigan and Arkansas- S1/S2, critically imperiled to imperiled; Colorado- S2, imperiled; Indiana, Iowa, and Wyoming- S3, vulnerable; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
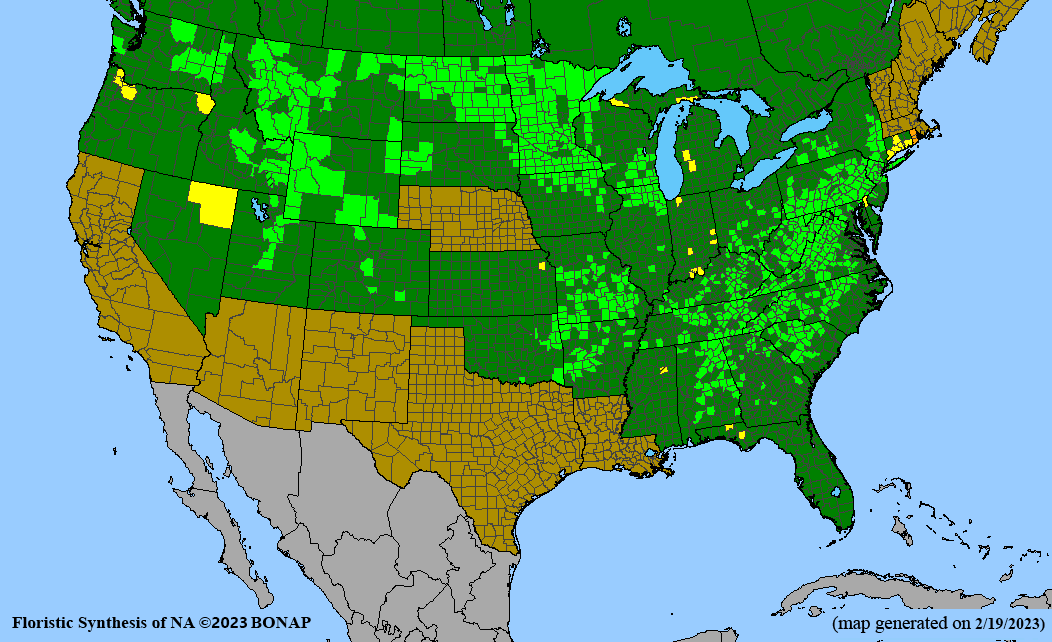
General Comments
The yellow umbellate flower heads of meadow zizia (Zizia aptera) closely resemble those of their cousin golden Alexanders (Z. aurea), but meadow zizia plants (also called heart-leaf Alexanders) have distinctive, heart-shaped lower leaves, generally occupy drier, more open habitats, and bloom a couple of weeks earlier in the spring. The early flowering time makes this species an important option for pollinator plantings, and their short stature may make these plants of interest for plantings in solar installations. The plants are long-lived in production plots and fairly trouble-free, but seed production declines after the first few years.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Seeds have underdeveloped embryos, and a lengthy cold/moist stratification (150 days) improves germination by providing time for embryos to develop while chemical changes also occur in the seed to reduce inhibition of germination.
Sowing: Sow in greenhouse, lightly covered with germination mix, about 2-3 months before the last frost. Seed germinates best at cooler temperatures (65-70°F, 18-21°C).
Transplanting: When seedlings form well-rooted plugs, move them outside to harden off, then transplant into prepared plasticulture beds at 8-12 in spacing.
- Stand management
Weeds: Plants are short-statured and vulnerable to competition from weeds. Prepare a clean, weed-free bed and use plastic mulch to suppress weeds in the first year or two. Mow or cultivate between rows. Hand weed or rogue to remove large or competitive weeds.
Pests: Aphids may infest the seed heads in large aggregations. While inspecting a plot Z. aptera, we have often observed several ebony bugs (genus Corimelaena) within a single developing seedhead. We suspect that these insects may reduce the yield of viable seed.
Diseases: None noted.
- Seed production
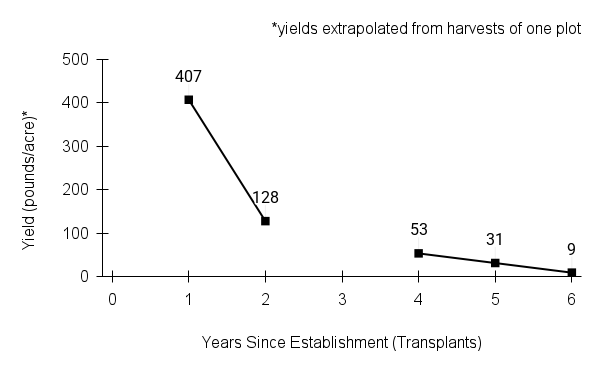 First harvest: Plants flower and set seed the year after planting. Peak harvest in our plot was also the first harvest.
First harvest: Plants flower and set seed the year after planting. Peak harvest in our plot was also the first harvest.Yield/acre: 50-400 pounds per acre (yields extrapolated from harvests of one plot at TPC)
Stand life: Plants are long-lived, but yields declined after peak harvest in year 2.
Flowering date: late April to early June in northern Iowa
Seed maturity/Harvest date: early to late August in northern Iowa
Seed retention: Fairly low risk of shattering.
Harvest date range at TPC (2015-2021): August 1 - August 28
Recommended harvest method: Hand pick or combine, depending on the size of planting and presence/absence of weed seeds that could contaminate the seedlot.
- Seed cleaning and storage
Cleaning process: Pass harvested material through 1/4 in mesh to remove larger particles, then airscreen.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern Iowa)
- References
Baskin, Jerry M.; Baskin, Carol C.. 2002. Propagation protocol for production of Container (plug) Zizia aptera (A. Gray) Fern. plants University of Kentucky Lexington, Kentucky. In: Native Plant Network. https://NativePlantNetwork.org (accessed 2025/12/30). US Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources.
Chayka, K. (n.d.). Zizia aptera (Heart-leaved Alexanders). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/heart-leaved-alexanders
Hilty, J. (2019). Heartleaf Golden Alexanders - Zizia aptera. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/heartleaf_alexx.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Moorman, S. (2021, July 18) Family Thyreocoridae - Ebony Bugs. Bug Guide, https://bugguide.net/node/view/6985
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
USDA NRCS National Plant Data Team. (n.d.). Zizia aptera (A. Gray) Fernald. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ZIAP
Species Guide Updated 12/30/2025
narrowleaf mountainmint
narrowleaf mountainmint dickeye
Pycnanthemum tenuifolium Schrad.
Alternate Common Name: slender mountain mint
Scientific Synonyms: Koellia flexuosa auct. non (Walter) MacMill., Pycnanthemum flexuosum auct. non (Walter) Britton, Sterns & Poggenb., Pycnanthemum linifolium
Family: mint family (Lamiaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Rhizomatous perennial that forms vegetative colonies.
Height: 1-3 ft
- Leaves and stem
Very narrow (1/8 in or less), opposite leaves with a strong mint/citrus odor when crushed; stems are slender, 4-sided, branched above, and entirely hairless, helping to distinguish this species from our other mountain mints.
- Flower, fruit and seedhead
Flower: Small (1/4 in long) two-lipped, white flowers with scattered purple dots, in clusters at stem tips.
Fruit/seed head: Seedheads are light brown when mature in contrast to the grey color of P. virginianum and P. pilosum seed heads.
Pollination: Insects such as bees, butterflies, wasps, and beetles
- Seed
Seed characteristics
Seeds per ounce: 378,000 (IA NRCS)
1000 seed weight: 0.08 g (Seed Information Database)
Description: Seed units are tiny nutlets (nearly 1 mm long), developing within each tube-like calyx of the inflorescence.
Typical seed test
PLS: 90% (n = 13)
Purity: 94% (n = 13)
Germination: 36% (n = 9)
Dormant: 45% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to dry-mesic soils in upland prairies; full sun; prairies, moist meadows, limestone glades, thickets, woodland openings, abandoned fields; Wetland Indicator Status is Facultative (FAC) for the Midwest.
Conservation status: Global- G5, secure; Nebraska- S1, critically imperiled (NatureServe)
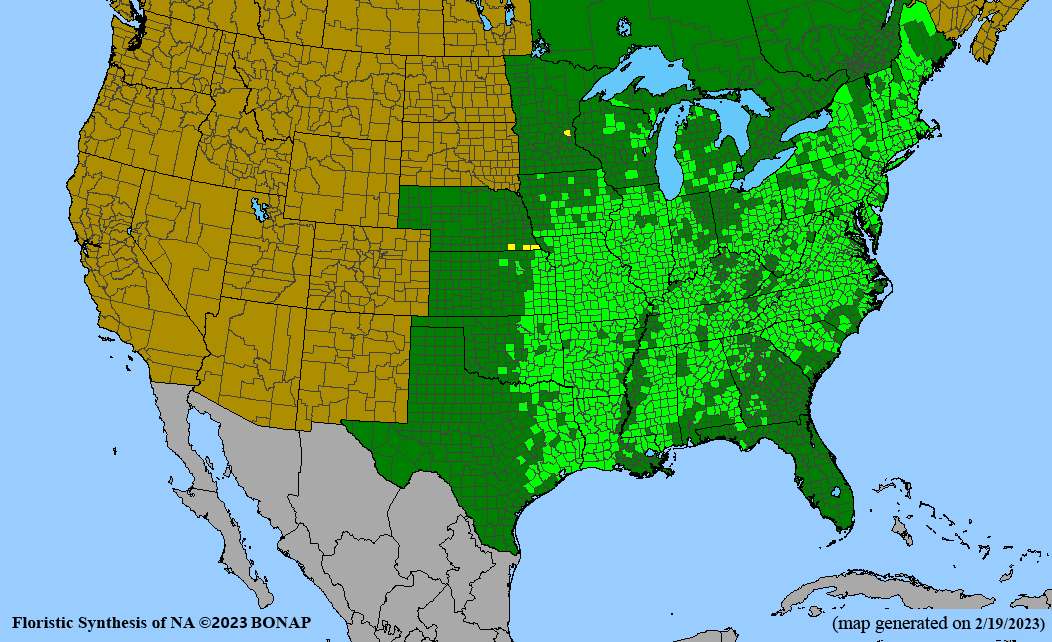
General Comments
Narrowleaf mountainmint, like other members of the genus Pycnanthemum, is visited by numerous and diverse pollinators including native bees, wasps, and flies. This species can be distinguished from the other members of the genus in Iowa by its hairless stems, narrower leaves (not exceeding 1/4 in), brown seed heads (not gray), and somewhat later flowering time. Plants are relatively easy to grow, yield heavily for a few years, and produce seed that stores well under refrigeration.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: Dry/cold stratify 12 weeks at 40° F.
Sowing: Surface sow seed in greenhouse two months before last frost free date. Water carefully (fine mist) to prevent seed from splattering out of containers.
Transplanting: Transplant into a weed barrier at 8-12 in intervals. Plants spread clonally, so the weed barrier can be removed by the third season, but seed production typically declines by the fourth season.
- Stand management
Weeds: Plastic mulch or weed barrier suppresses many weeds during the first year or two. Hand rogue weeds, being careful not to uproot seedlings or disturb roots and rhizomes of the mountainmint. An anecdotal report from a commercial native seed grower suggests that cultivation within mountainmint rows weakens plants and can cause loss of the crop.
Pests: None noted.
Diseases: None noted.
- Seed production
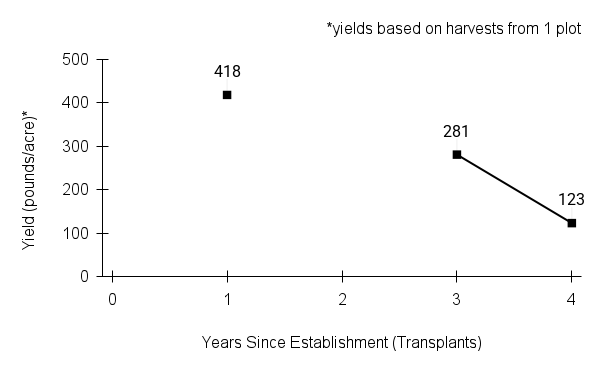 First harvest: Some flowering and seed production first year from greenhouse grown transplants.
First harvest: Some flowering and seed production first year from greenhouse grown transplants.Yield: 10-80 bulk pounds/acre
Stand life: Peak harvests second-third year. Stand persists but seed production may decline significantly fourth year and after.
Flowering date: Flowering occurs mid-July into August.
Seed maturity/Harvest date: Mid September to early October.
Seed retention: Holds seed well, shattering occurs mid to late October.
Harvest date range at TPC (2004-2007): Sept 21 - Oct 18
Recommended harvest method: Combine, no air.
- Seed cleaning and storage
Cleaning process: Pre-clean by scalping thru 1/2 in and 1/4 in mesh to remove large particles and make flowable, then air-screen. (No awns or appendages to remove).
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 3 (southern Iowa)
- References
Gleason, H. A., & Cronquist, A. (1991). Lamiaceae. In Manual of Vascular Plants of Northeastern United States and Adjacent Canada (2nd ed., p. 443). The New York Botanical Garden.
Hilty, J. (2019). Slender mountain mint - Pycnanthemum tenuifolium. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/slm_mintx.htm
Houseal, G. A. (2007). Forbs Wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 44–45). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Pycnanthemum tenuifolium Schrad. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=PYTE
Species Guide Updated 12/5/2025
pale purple coneflower
pale purple coneflower dickeye
Echinacea pallida (Nutt.) Nutt.
Alternate Common Names: prairie coneflower, pale echinacea
Scientific Synonyms: Brauneria pallida (Nutt.) Britton, Rudbeckia pallida Nutt.
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from a stout taproot.
Height: 2-3 ft
- Leaves and stem
Leaves mostly basal, long tapered, coarsely hairy with three prominent parallel veins; stems grayish to reddish green, covered in coarse hairs, unbranched.
- Flower, fruit and seedhead
Flower: Single composite flower head at top of stem with 7 or more pink-purple (sometimes white), slender, drooping ray florets and a prominent central cone. Pollen is white.
Fruit/seedhead: Seed head is a dark brown-black cone, about 1 inch in diameter. Seeds (achenes) develop from disk flowers, which bloom from the outer ring of the cone inwards.
Pollination: Insects, primarily bees and butterflies.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 5,200 (IA NRCS)
Seeds per pound: 83,200 (IA NRCS)
1000 seed weight: 5.66 g (Seed Information Database)
Description: The “seed unit” is a dry fruit called an achene, about 1/8 in long, with a dark brown band at the square end and pale gray from there to the pointed end. No awns or appendages to remove.
Typical seed test
PLS: 96% (n = 10)
Purity: 99% (n = 10)
Germination: 44% (n = 9)
Dormant: 52% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry-mesic to mesic soil; prefers sites with well-drained upland soils and full sun; prairies, oak savannas, abandoned fields, dry woodland openings, along railroads. Avoid wet or poorly drained soils for seed production.
Conservation status: Global- G4, apparently secure; Georgia, Nebraska, North Carolina, and Tennessee- S1, critically imperiled; Alabama- S2, imperiled; Wisconsin- S3, vulnerable (NatureServe)
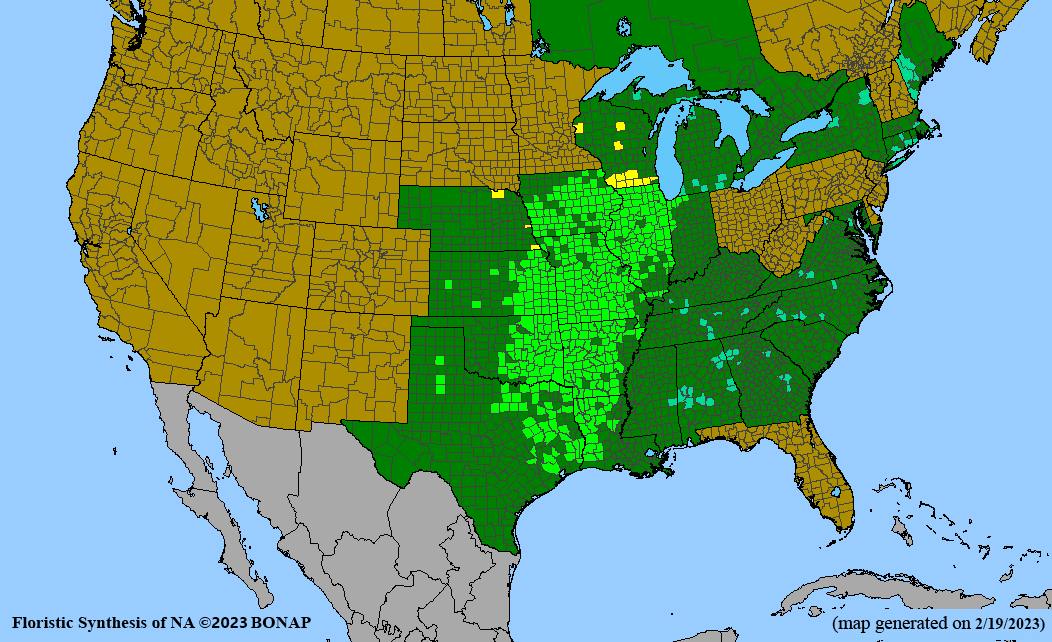
General Comments
This species is best propagated in the greenhouse and transplanted into a weed barrier or bare soil. Weed suppression is essential for good establishment and seed production. Combine harvest is fairly straightforward, since it retains seed well in the heads. All Echinacea species are known to hybridize, so proper isolation should be maintained between related species to prevent hybrid seed production (McGregor 1968).
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 3.0-5.0
Seeds per linear foot: 40
Seeding depth: 1/8 in
Seeding method: native seed drill
Seeding time: dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Wet stratify 12 weeks at 40° F.
Sowing: Sow seed surface to 1/8 in deep in the greenhouse two months before last frost free date. Literature suggests this species needs light to germinate, but covering seed lightly to 1/8 in does not inhibit germination.
Transplanting: When root growth is sufficient to produce a sturdy plug, harden off, then transplant into bare soil in rows or weed barrier at 8-12 in intervals after all danger of frost.
- Stand management
Weeds: Planting into a weed barrier or plastic mulch suppresses weeds in the first growing season; hand rogue weeds, being careful not to uproot seedlings.
Pests: None noted.
Diseases: None noted.
Hybridization risk: Echinacea angustifolia, E. purpurea
- Seed production
 First harvest: Second year plants.
First harvest: Second year plants.Yield: 55-210 bulk pounds/acre (yields extrapolated from harvests of 6 plots)
Stand life: Peak harvests second year. Good harvest third year. Stand persists, but seed production declines significantly fourth year and after.
Flowering date: mid-June to mid-July in northern Iowa
Seed maturity/Harvest date: September in northern Iowa
Seed retention: Generally holds seed well, some shattering may begin in late September and extend throughout the winter months.
Harvest date range at TPC (2003-2021): Aug 5 - Oct 25
Recommended harvest method: Combine before significant shattering occurs.
- Seed cleaning and storage
Cleaning process: Combine does a superb job of threshing seedheads. Pre-clean combined material by scalping through 1/2 in and 1/4 in mesh to remove large particles and make flowable, then air-screen. If hand harvested, seedheads need to be threshed using a hammermill or brush machine, using care not to overclean and damage the hulls of the achenes.
Seed storage: Cool/dry (33-50° F, 30-50% RH).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zones 1, 2, and 3; Northern Missouri Germplasm, Western Missouri Germplasm
- References
Chayka, K. (n.d.). Echinacea pallida (pale purple coneflower). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/pale-purple-coneflower
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Purple coneflowers. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 74–75). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Pale purple coneflower - Echinacea pallida. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/pale_coneflowerx.ht…;
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 32–33). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Echinacea pallida (Nutt.) Nutt. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ECPA
Species Guide Updated 12/3/2024
parasol whitetop
parasol whitetop sagem
Doellingeria umbellata (Mill.) Nees
Alternate Common Names: flat-top aster, parasol aster, tall flat-topped white aster
Scientific Synonyms: Aster umbellatus Miller, Diplopappus umbellatus (Miller) Hooker, Diplostephium umbellatum (Miller) Cassini
Family: aster or sunflower family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Long-lived perennial, spreads by short rhizomes to form small colonies.
Height: 1-5 ft

- Leaves and stem

Lance-shaped, 3-5 in long and 1/2-1 in wide, leaf margins toothless, leaf surfaces usually hairless or with short hairs, alternate arrangement; stems are erect and unbranched except within the flower head, with few to no hairs, generally light yellowish-green but sometimes purple.
- Flower, fruit and seedhead
Flower: Individual heads are daisy-like, about 1/2 in wide, with yellow to tan centers and usually 5-10 (up to 15) white “petals” (rays) irregularly arranged around the central disc; dozens to hundreds of heads in a branched, flattened cluster up to 10 or 12 in wide.
Fruit/seedhead: Seed head appears fuzzy due to creamy-white fluff (pappus) on seeds.
Pollination: Insects, including bees, butterflies, and moths

- Seed
Seed characteristics
Seeds per ounce: 67,000 (IA NRCS)
1000 seed weight: 0.7 g (Seed Information Database)
Description: Seed (achene) is light brown, 2.5-3 mm long, approximately 1 mm wide near the top, tapered to a point on the lower end, and bears a “parachute” of creamy white fluff (pappus).
Typical seed test
PLS: 85% (n = 5)
Purity: 90% (n = 5)
Germination: 22% (n = 4)
Dormant: 71% (n = 4)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soils, preferably with some sand; partial to full sun; wet prairies, sedge meadows, seasonally wet ditches, and fens; benefits from irrigation in production systems. The USDA classifies it as a Facultative Wetland species in the Midwest region.
Conservation status: Global- G5, secure; Delaware, Iowa, and North Carolina- S3, vulnerable (NatureServe)
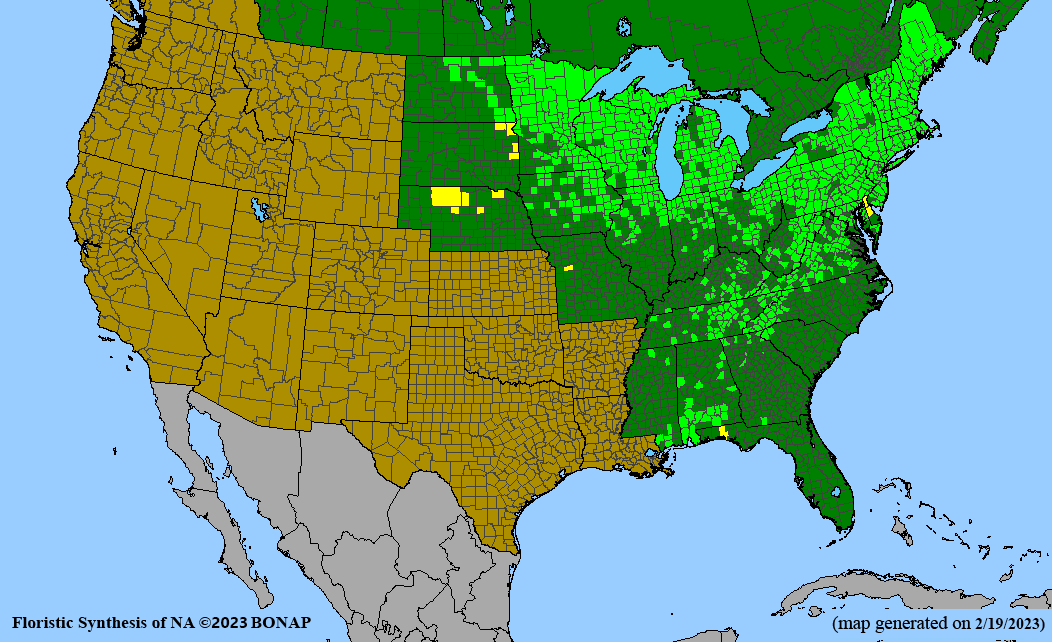
General Comments
Parasol whitetop is a late season nectar and pollen source for diverse species of bees (including specialist bees), wasps, beetles, flower flies, and skipper butterflies. It is a larval host to some species of checkerspot and crescent butterflies. It can grow and flower for many years in mesic soils but benefits from irrigation in production systems.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: 60 days cold-moist stratification results in uniform and high rates of germination.
Sowing: Seed is small and should be only lightly covered with growing media. If started in germination flats, transplant to individual plugs when seedlings have their first pair of true leaves, about 2 weeks after seeding.
Transplanting: Seedlings are ready to transplant to the field about 6-8 weeks after transferring them to plugs, when their roots are well-branched and numerous root tips are visible at hole(s) in the base of the plug. Pop out a few plugs to check for adequate root development that will provide sturdy plugs for planting. Seedlings are fast growing and may need to be clipped back before transplanting to improve the shoot:root ratio. A week or two before transplanting, move flats outside to ‘harden off.’ (See Propagation chapter in General Information for more details).
- Stand management
Weeds: In the first season after transplanting, weeds are suppressed by a plastic weed barrier. Plants spread slowly by short rhizomes; in second and subsequent years, holes in plastic may need to be expanded or plastic removed to make room for new stems.
Pests: None noted.
Diseases: None noted.
Soil moisture: This species benefits from drip irrigation when planted in mesic soils.
- Seed production
 First harvest: Plants will flower and produce a small amount of seed in the planting year when started from transplants.
First harvest: Plants will flower and produce a small amount of seed in the planting year when started from transplants.Yield: 28-74 pounds/acre (based on 1 plot)
Stand life: unknown
Flowering date: August - September
Seed maturity/Harvest date: late September - late October
Seed retention: Seeds are wind dispersed soon after maturity, when fluff (pappus) expands in late September through October.
Harvest date range at TPC (2022-2023): Sept 15 - Oct 20
Recommended harvest method: Seed is released from heads within days of the fluffy “parachutes” expanding. Watch for the centers of seed clusters to begin shattering, and pick early maturing seed heads (clip stalks below seed clusters). If some heads in a cluster are still closed (not fluffy), pull apart a few heads to see if the seeds are dark colored and separate easily from the base (receptacle). Combine the rest of the plot at peak maturity. Turn off air or combine will disperse the fluffy seeds.
- Seed cleaning and storage
Cleaning process: If hand clipped, run dried material through a 1/4 in mesh to thresh seed from stalks. Use a brush machine (medium bristles, low vacuum) to remove pappus. May need two rounds of brushing. Winnow with a box fan to separate seed from most of the pappus and chaff. Airscreen 2-3 times, then indent to remove broken bits of stems. See Appendix C for specific settings.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI

- References
Chayka, K. (n.d.). Doellingeria umbellata (flat-topped white aster). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/flat-topped-white-aster
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Flat-top aster. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 133). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Flat-topped aster - Doellingeria umbellata. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/fltp_aster.html
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
USDA NRCS National Plant Data Team. (n.d.). Doellingeria umbellata (Mill.) Nees. USDA plants database. https://plants.usda.gov/plant-profile/DOUM2
Van Der Grinten, Martin. (2001). Propagation protocol for production of Container (plug) Aster umbellatus P. Mill. plants USDA NRCS - Big Flats Plant Materials Center Corning, New York. In: Native Plant Network. URL: https://NativePlantNetwork.org (accessed 2024/02/02). US Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources.
Semple, J. C., & Chmielewski, J. G. (2020, November 6). Doellingeria umbellata (Miller) Nees. Flora of North America. http://floranorthamerica.org/Doellingeria_umbellata
Species Guide Updated 12/20/2024
pinnate prairie coneflower
pinnate prairie coneflower dickeye
Ratibida pinnata (Vent.) Barnhart
Alternate Common Names: gray-headed coneflower, yellow coneflower, globular coneflower, drooping coneflower, gray coneflower, prairie coneflower, weary susan, grayhead coneflower, drooping yellow coneflower
Scientific Synonyms: Lepachys pinnata (Vent.) Torr. & A. Gray, Rudbeckia pinnata Vent.
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial that forms tight clumps or colonies.
Height: 3-7 ft
- Leaves and stem
Leaves are alternate, irregular in shape and pinnately divided into 3-7 lobes, with short, rough hairs; stems are long, stiff, and slender, unbranched except near the top.
- Flower, fruit and seedhead
Flower: Composite flower heads borne singly at the tips of long stalks with drooping 1 in long yellow petals (rays) surrounding the egg-shaped dome of disk flowers.
Fruit/seed head: Firm, dense seedheads often hold some seed into late fall and winter and release an anise or citrus scent when crushed.
Pollination: Insects including bees, butterflies, flies, wasps, and beetles.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 30,000 (IA NRCS)
1000 seed weight: 0.87 g (Seed Information Database)
Description: Seeds (achenes) develop from fertile disc flowers. Achenes are brown to black and about 1/16 in long.
Typical seed test
PLS: 93%
Purity: 99%
Germination: 76%
Dormant: 8%
(averages obtained from 11 tests of purchased seed lots)
- Habitat and range
Habitat: Wet-mesic to dry-mesic loamy soils; full sun; usually common on medium to high quality prairies; remnant prairies, thickets, woodland edges, prairies, limestone glades.
Conservation status: Global- G5, secure; West Virginia- SH, possibly extirpated; Pennsylvania and South Carolina- S1, critically imperiled; Florida and Louisiana- S2, imperiled (NatureServe)
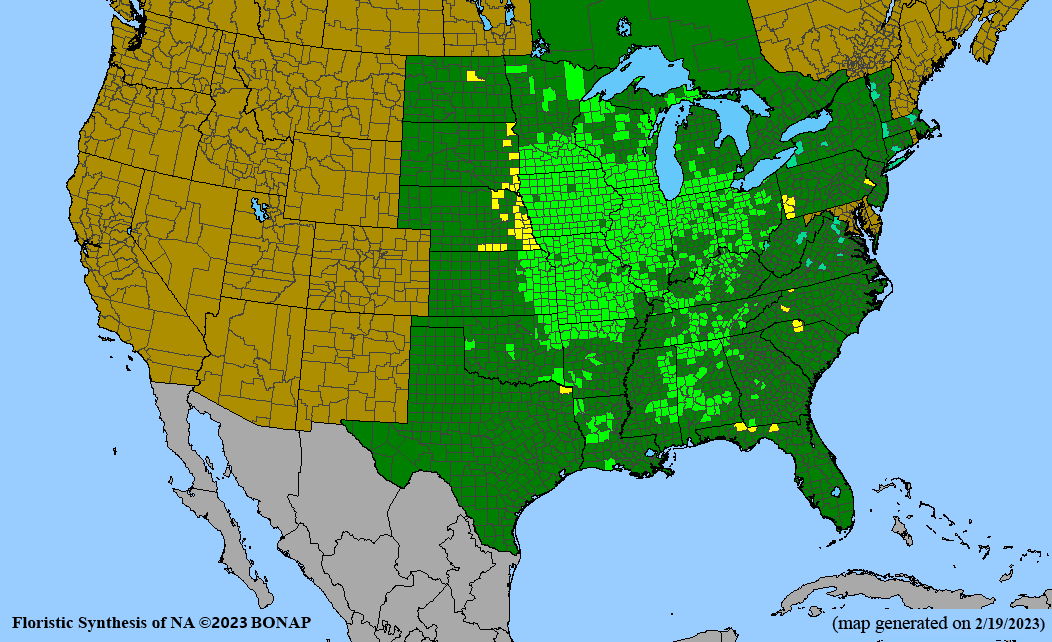
General Comments
Pinnate prairie coneflower is typically common on Midwestern prairies and establishes readily from seed in reconstructed prairies. Some seeds generally stay in the seedheads through fall and into winter are eaten by songbirds. Seed harvesting and cleaning are relatively straightforward if good weed control is maintained.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 3.6-5.0
Seeds per linear foot: 40
Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Wet stratify 8-12 weeks at 40° F.
Sowing: Sow seed in greenhouse two months before last frost free date.
Transplanting: Harden-off, transplant into bare soil in rows or weed barrier at 8-12 in intervals after all danger of frost.
- Stand management
Weeds: Mow/cultivate between rows. Post emergence grass herbicide, tillage, roguing.
Pests: None noted.
Diseases: None noted.
- Seed production
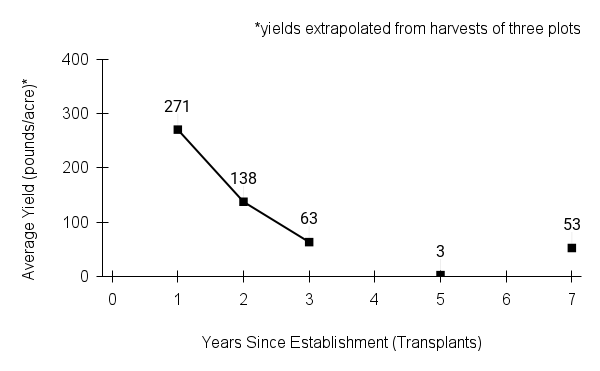 First harvest: Remain vegetative the first year, abundant flowering and seed production second year.
First harvest: Remain vegetative the first year, abundant flowering and seed production second year.Yield: 100-250 bulk pounds/acre
Stand life: Peak harvests second year. Good harvest third year. Stand persists but seed production may decline significantly fourth year and after.
Flowering date: Flowering occurs early July to mid-August.
Seed maturity/Harvest date: Late September
Seed retention: Shattering occurs mid to late October.
Harvest date range at TPC (2004-2010): Sept 3 - Oct 27
Recommended harvest method: combine
- Seed cleaning and storage
Cleaning process: Pre-clean by scalping through 1/2 in and 1/4 in mesh to remove large particles and make flowable, then air-screen. Foxtail can be removed from most of the seed with scalping screens, followed by a final cleaning with a belt-sorter or velvet roller of scalped material. (Brushing is not needed as there are no awns or appendages to remove).
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zones 1 (northern Iowa), 2 (central Iowa), and 3 (southern Iowa); Northern Missouri Germplasm
Cultivated variety (cultivars): Sunglow (KS)
- References
Chayka, K. (n.d.). Ratibida pinnata (gray-headed coneflower). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/gray-headed-coneflower
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Yellow coneflower. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 105). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Yellow coneflower - Ratibida pinnata. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/yl_coneflowerx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 48–49). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Yellow coneflower. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 154–155). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Ratibida pinnata (Vent.) Barnhart. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=RAPI
Species Guide Updated 12/5/2025
prairie blazing star
prairie blazing star dickeye
Liatris pycnostachya Michx.
Alternate Common Names: cat-tail gayfeather, thick-spike gayfeather, prairie blazingstar, prairie blazing-star, button snakeroot, gayfeather, blazing star, thick-spike gay-feather, thick-spike blazing-star
Scientific Synonyms: Lacinaria pycnostachya (Mich.) Kuntze
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from corms that can be divided.
Height: 2-5 ft

- Leaves and stem
Leaves grasslike, alternate but so crowded as to appear almost whorled, becoming progressively shorter toward the top of the stem; stem is hairy and stiff, unbranched.
- Flower, fruit and seedhead
Flower: Heads of 5-10 purplish-pink disk flowers with long styles that stick out; slender heads are densely packed in spikes that bloom from the top down.
Fruit/seedhead: “Seed” is an achene bearing a tan pappus (tuft of hair), distributed by the wind.
Pollination: Insects such as butterflies, bees, and moths.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 11,000 (IA NRCS)
1000 seed weight: 1.86 g (Seed Information Database)
Description: “Seeds” are achenes, about 1/8 to 1/4 in long, with tufts of tan hairs (pappus).
Typical seed test
PLS: 93% (n = 10)
Purity: 98% (n = 10)
Germination: 22% (n = 7)
Dormancy: 73% (n = 6)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to wet-mesic soils; full sun; may be abundant in medium to high quality prairies; moist meadows, rocky bluffs, along railroads, limestone glades. Wetland Indicator Status is Facultative (FAC) for the Midwest. Moist, but well-drained soils are preferred for seed production.
Conservation status: Global- G5, secure; Indiana and South Dakota- S1, critically imperiled; (NatureServe)
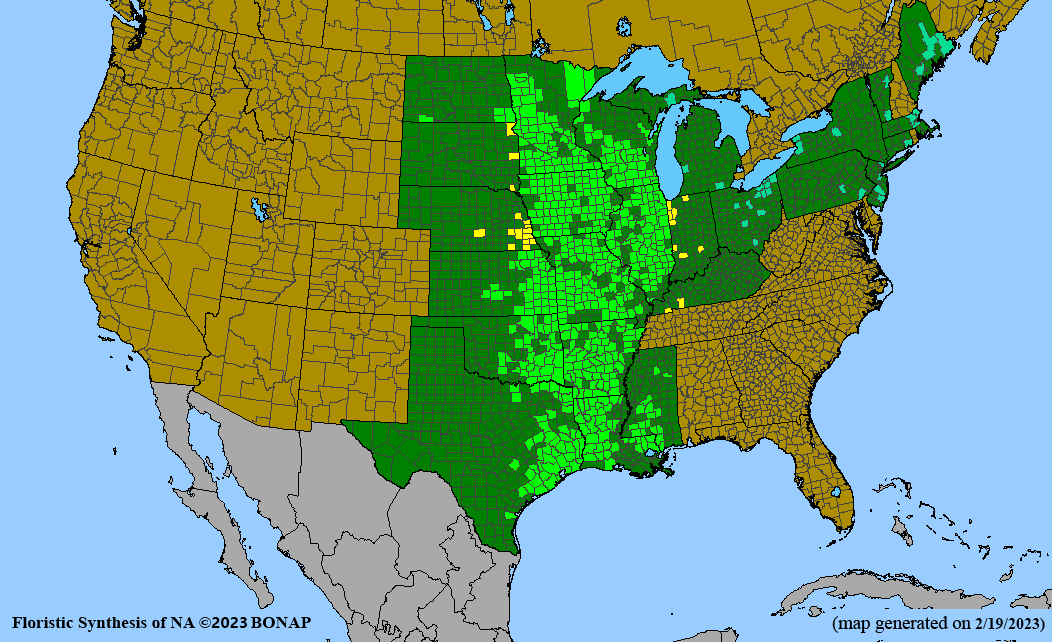
General Comments
Prairie blazing star is best propagated in the greenhouse, where seedlings form small pea-sized corms after about 2 months. First true leaves of seedlings are grass-like. Expect prolific seed production in the second growing season, after which the stand declines. Corms can be dug and divided for fall transplant, if disease free, for abundant flowering and seed set the following growing season. Species in the genus Liatris are known to hybridize, therefore proper isolation should be maintained between related species to avoid hybrid seed production (Levin 1968, Menhusen 1972). Liatris species and cultivars are also produced commercially for the landscaping and cut-flower industries.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species
Greenhouse
Seed pre-treatment: Wet stratify 8-12 weeks at 40˚ F. Seed may be prone to mold in stratification. Some growers add fungicide to the stratification media.
Sowing: Sow seed in greenhouse two months before last frost free date.
Transplanting: Harden-off, transplant into bare soil in rows or weed barrier at 8 in intervals after all danger of frost.
- Stand management
Weeds: Mow/cultivate between rows, mulch within rows. Post emergence grass herbicide, tillage, hoeing, hand roguing. Very sensitive to soil disturbance during bolting/flowering, so clip weeds rather than pulling or hoeing after flowering shoots emerge.
Pests: Voles will eat and/or cache corms, rabbits and deer eat young shoots, goldfinches eat seed as it matures.
Diseases: powdery mildew, root-knot nematodes, stem rot, verticillium wilt.
Hybridization risk: This species is known to hybridize with related species Liatris acidota, L. ligulistylis, L. punctata, and L. squarrosa
- Seed production
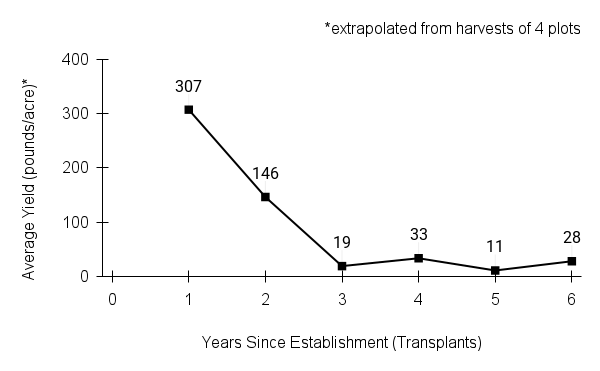 First Harvest: Plants remain vegetative for the first year (seedlings); abundant flowering/seed production second year. Fall corm division/transplanting results in abundant flowering the following growing season.
First Harvest: Plants remain vegetative for the first year (seedlings); abundant flowering/seed production second year. Fall corm division/transplanting results in abundant flowering the following growing season.Yield: 11-307 pounds/acre (per acre yields extrapolated from harvest records of 4 plots)
Stand life: Peak harvests occur in the second year. Good harvest third year if proper soils. Stand declines significantly fourth year and after. Plants tend to lodge second year when flowering. On more well-drained soils, irrigation may improve yields.
Flowering date: late July - late August in northern Iowa
Seed maturity/Harvest date: early September - mid-October in northern Iowa
Seed retention: Seed is wind dispersed shortly after ripening when pappus “parachutes” dry and fluff out
Harvest date range at TPC (2003-2023): Sept 2 - Oct 23
Recommended Harvest Method: Combine at maturity, but before pappus is dry and fluffy. Seedheads mature from the top down along a stalk. When the topmost heads are fluffy, break open a few of the lower heads and observe for signs of maturity: dark-colored seeds that separate easily from the base of the head. Small plots may be hand harvested by clipping stalks as the seed matures, then drying the cut material in a building. Dry seed threshes easily from stalks.
- Seed cleaning and storage
Cleaning process: Pre-clean by scalping thru 1/2 in mesh to remove large particles and make the material flowable, brush gently with soft bristles to remove fluffy pappus, using care not to damage seed coat, then air screen.
Seed storage: cool/dry (33-50° F, 30-50% RH); seed stores well for a few years if seed is not damaged during cleaning.
Released Germplasm
Source Identified material: Northern Iowa Germplasm (IA Zone 1), Central Iowa Germplasm (IA Zone 2), Southern Iowa Germplasm (IA Zone 3), Northern Missouri Germplasm, Western Missouri Germplasm
Selected Germplasm: Pineywoods Germplasm (TX)
Cultivated variety (cultivars): Eureka (KS; developed for cut flower industry)
- References
Chayka, K. (n.d.). Liatris pycnostachya (prairie blazing star). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/prairie-blazing-star
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Thick-spike gay-feather. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 98). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Prairie blazingstar - Liatris pycnostachya. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/pr_blazingstarx.htm
Houseal, G. A. (2007). GForbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 40–41). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Levin, D.A. (1968). The Structure of a Polyspecies Hybrid Swarm in Liatris. Evolution, 22(2), 352-372. https://doi.org/10.1111/j.1558-5646.1968.tb05903.x
Menhusen, B.R. (1972). Ecology of the Prairie Species of the Genus Liatris. Third Midwest Prairie Conference Proceedings. Manhattan, Kan.: Division of Biology, Kansas State University. https://search.library.wisc.edu/digital/AL7JMUVRYYXDZO8S/pages/A56MVY3FXXELEL8L
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Nesom, G. L. (2020, November 6). Liatris pycnostachya Michaux. Flora of North America. http://floranorthamerica.org/Liatris_pycnostachya
Runkel, S. T., & Roosa, D. M. (2009). Blazing star. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 198–199). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Liatris pycnostachya Michx. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=LIPY
Species Guide Updated 2/21/2025
showy goldenrod
showy goldenrod dickeye
Solidago speciosa, Nutt.
Alternate Common Names: goldenrod
Scientific Synonyms: Aster speciosus (Nutt.) Kuntze
Family: Aster Family, Daisy Family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from a fibrous root system; clumps of stems expand slowly from rhizomes.
Height: 1 - 5ft

- Leaves and stem
Leaves alternate; basal leaves up to 12 in long and 3 in wide, leaf size decreasing up the stem; leaves lance-shaped to oval with smooth margins, mostly hairless; stem green to reddish purple, unbranched.
- Flower, fruit and seedhead
Flower: Inflorescence of numerous small, bright yellow composite heads is more upright than those of most other goldenrods, shaped like an elongated, inverted cone at the end of the stem; individual heads (1/4 in wide) are smaller than those of stiff goldenrod (Oligoneuron rigidum) but larger than those of more common “weedy” goldenrods (e.g., Solidago canadensis).
Fruit/seed head: Heads become fluffy as the pappus on the ripening achenes expands.
Pollination: Insects, especially bees, wasps, and beetles, but also butterflies, moths, and flies.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 95,000 (IA NRCS)
1000 seed weight: 0.26g (Seed Information Database)
Description: “Seed” is a small achene, 2 mm long and less than 1 mm wide, pale tan with darker, shallow grooves, tufted with a pale pappus for wind dispersal.
Typical seed test
PLS: 89% (n = 12)
Purity: 93% (n = 12)
Germination: 19% (n = 9)
Dormancy: 70% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic soil; partial to full sun; sand prairies, along railroads, thickets, woodland edges, rocky upland forest openings, savannas, abandoned fields. Make sure that production plots are sited in well-drained mesic to dry-mesic soils as these plants perform poorly if soils remain saturated for an extended period.
Conservation status: Global- G5, secure; Vermont- SH, possibly extirpated; Maine- S1, critically imperiled; Maryland, Ohio, and Wyoming- S2, imperiled; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
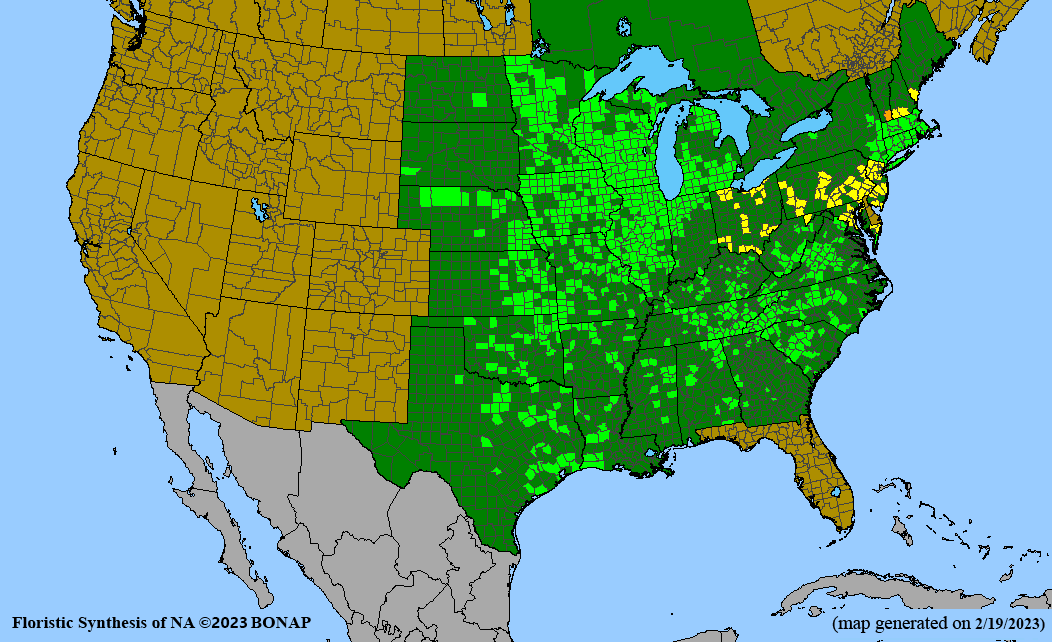
General Comments
The name “showy goldenrod” suits this species well. Its upright wands of bright yellow heads shine like torches in the prairie for a month in late summer and fall. While rhizomatous, this species spreads less aggressively than the more common, clonal goldenrod species like Canada goldenrod, and it tends to be found in drier habitats. Pollinators of many kinds, including migrating monarch butterflies and the endangered rusty patched bumble bee, are drawn to the pollen and nectar it provides late into the fall. There are subspecies of S. speciosa that have been elevated to species level fairly recently, and we are not yet certain how this may affect how showy goldenrod is grown and marketed for the native seed supply.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Cold/moist stratification for 60 days works well.
Sowing: Surface sow (or cover very lightly) in germination flats or plugs (2-3 seeds per cell) in the greenhouse about 2-3 months before the last frost. We find that 73-cell plug flats with deep cells, grooved sides, and wide bottom openings encourage the formation of well-rooted plugs.
Transplanting: When plugs are well-rooted, move the flats outside to harden off, then transplant at 8-12 in intervals in prepared plasticulture rows in well-drained soil after danger of frost.
- Stand management
Weeds: Prepare a clean, weed-free bed and use plastic mulch to reduce weed pressure in the first year. Remove plastic at the end of the first growing season to prevent moisture issues. Mow or cultivate between rows. Hand weed or rogue to remove competitive weeds or those that would contaminate the seed crop (e.g., small seeded asters or other goldenrods).
Pests: None noted.
Diseases: If soil moisture is excessive, plants succumb to root diseases. We have not identified the particular pathogens involved but have lost large numbers of plants in a plot due to saturated soil conditions during a protracted wet spring. Site production plots for this species in very well-drained locations.
- Seed production
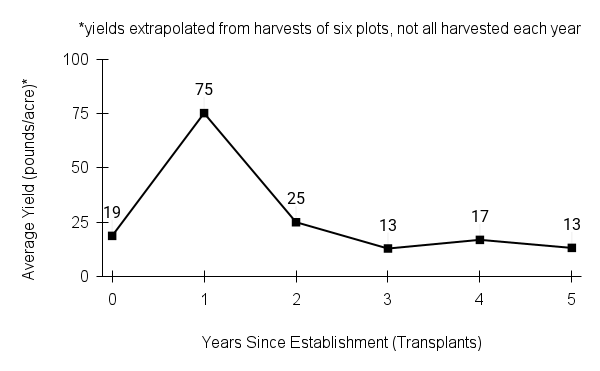 First harvest: A small harvest can be expected in the first year from transplants. Peak harvest is in the second year.
First harvest: A small harvest can be expected in the first year from transplants. Peak harvest is in the second year.Yield/acre: 20-75 pounds per acre (extrapolated from harvests of six plots grown at TPC)
Stand life: Plants may persist for many years in suitable soils, but in our experience, seed yields peaked in the year after planting, then declined in year 3 and remained low.
Flowering date: late August to late September in northern Iowa
Seed maturity/Harvest date: early to late October in northern Iowa
Seed retention: High risk of shattering as soon as pappus is fluffy.
Harvest date range at TPC (2004-2024): October 4 - October 25 (later harvests possible in planting year)
Recommended harvest method: Hand clip early maturing heads just as pappus begins to be visible at the tips of ripening heads, then combine the plot at peak maturity. Swathing, then ripening the stems in a sheltered location such as a covered shed, might reduce loss of ripe seed to the wind.
- Seed cleaning and storage
Cleaning process: Pass harvested material through 1/4 in mesh to remove larger pieces of stems and leaves. (If hand harvested, this step serves to thresh the seeds from the stems.) Brush with medium bristles to remove pappus from achenes, then air-screen at least two times.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern Iowa), Zone 2 (central Iowa), and Zone 3 (southern Iowa).
- References
Chayka, K. (n.d.). Solidago speciosa (Showy Goldenrod). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/showy-goldenrod
Hilty, J. (2019). Showy Goldenrod - Solidago speciosa. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/shw_goldenrodx.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Missouri Botanical Garden. (n.d.). Solidago speciosa. Missouri Botanical Garden - Plant Finder https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=277530&isprofile=0&This
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Semple, J. C., & Cook, R. E. (2020, November 5). Solidago speciosa Nuttall. Flora of North America. http://floranorthamerica.org/Symphyotrichum_oolentangiense
USDA NRCS National Plant Data Team. (n.d.). Solidago speciosa Nutt. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SOSP2
Species Guide Updated 1/5/2026
smooth oxeye
smooth oxeye sagem
Heliopsis helianthoides (L.) Sweet
Alternate Common Names: common ox-eye, false sunflower, sunflower heliopsis, ox-eye, sunflower-everlasting
Scientific Synonym: Buphthalmum helianthoides L.
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial, fibrous rooted, producing clumps or colonies from rhizomes.
Height: 2-6 ft
- Leaves and stem

Leaves opposite, egg-shaped with pointed tips, saw-tooth margins, rough texture, up to 5 in long and 3.5 in wide; stem is erect, rough with short hairs, branched.
- Flower, fruit and seedhead
Flower: One to 15 sunflower-like yellow flower heads, 1.5-3 in (4-7.5 cm) in diameter, at ends of long stalks from stem tip and upper leaf axils; ray florets are yellow-orange color, center disk usually golden yellow; underside of the flower head with alternating short and long bracts (phyllaries).
Fruit/seedhead: Flower head matures to a head of “seed” (achenes); achenes are dark, 3-4 angled, 4-5 mm long, and lack pappus; both disk and ray florets are fertile and produce achenes.
Pollination: Insects such as bees, wasps, beetles, flies, and butterflies.
- Seed
Seed characteristics
Seeds per ounce: 6,300 (IA NRCS)
Seeds per pound: 100,800 (IA NRCS)
1000 seed weight: 4.15 g (Seed Information Database)
Description: Seed unit is a smooth, dark achene about 3/16 in (4-5 mm) long.
Typical seed test
PLS: 95% (n = 10)
Purity: 100% (n = 10)
Germination: 65% (n = 9)
Dormant: 31% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry-mesic to wet-mesic soil; full sun; low to high quality remnant prairies, disturbed areas, roadsides, railroads, woodland openings and edges, thickets, streambanks, limestone glades. Wetland Indicator Status is Facultative Upland (FACU) for the Midwest.
Conservation status: Global- G5, secure; Delaware- S1, critically imperiled; Louisiana- S3, vulnerable (NatureServe)
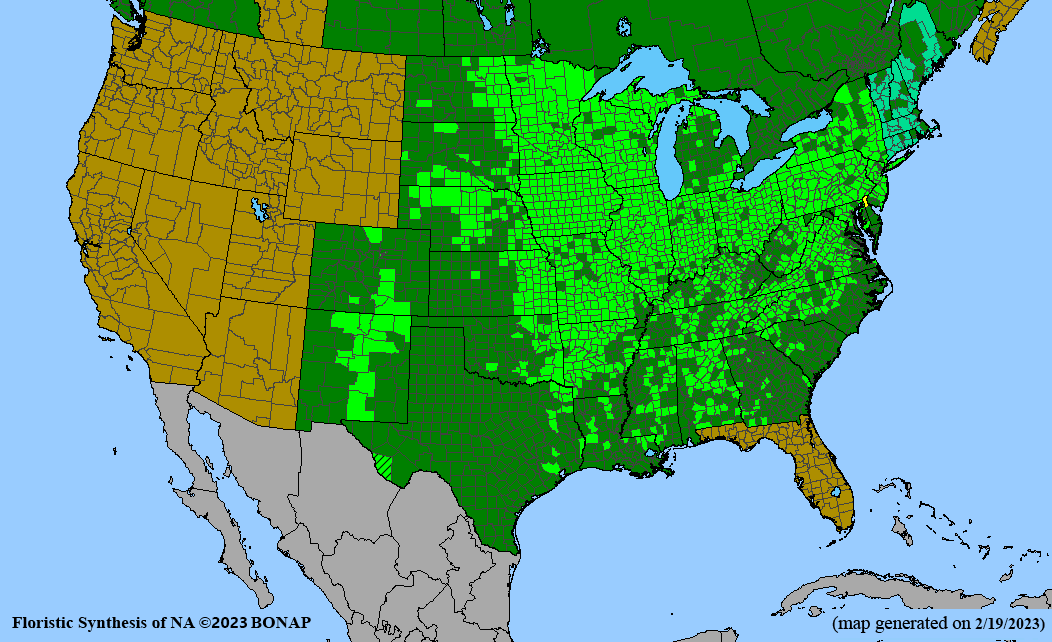
General Comments
Smooth oxeye is a component of most tallgrass prairies with medium to dry soils where it grows in association with tall, warm-season grasses. The long flowering time and abundant nectar and pollen make this an important pollinator resource, and the nutritious seeds are eaten by birds and mammals. This species is fairly easy to establish by direct seeding, if good seedbed preparation and weed suppression are achieved. Extended flowering and seed-ripening period makes determining optimal combine harvesting time difficult. Seed cleaning is accomplished with air-screen cleaning.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 4.0
Seeds per linear foot: 40
Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Wet stratify 8-12 weeks at 40° F.
Sowing: Sow seed at 1/4 in depth about two months before the last frost-free date.
Transplanting: Harden off, transplant into bare soil in rows or weed barrier at 8 in intervals after all danger of frost. Since this species spreads by rhizomes to form clumps, remove weed barrier after establishment year or use biodegradable barrier.
- Stand management
Weeds: Mow/cultivate between rows. Post emergence grass herbicide, tillage, roguing.
Pests: Plants may be affected by red aphids (Uroleucon) though these infestations are often cleared by parasitic wasps and aphid predators, and it is not clear that they cause much harm to the plants.
Diseases: Powdery mildew.
- Seed production
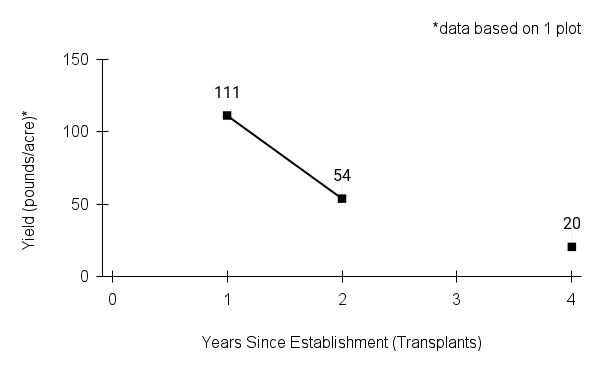
First harvest: Some flowering and seed production in first growing season from transplants and in well-managed direct seeded stands.
Yield: 20-115 pounds/acre (per acre yield extrapolated based on production from 1 plot)
Stand life: Peak harvest second-fourth year with declining yields in subsequent years.
Flowering date: early June - late July in northern Iowa
Seed maturity/Harvest date: mid-August to late September in northern Iowa; complicated by long flowering and seed ripening period.
Seed retention: Shattering occurs mid to late October.
Harvest date range at TPC (2005-2008): Sept 15 - 21
Recommended harvest method: Combine
- Seed cleaning and storage
Cleaning process: Pre-clean by scalping through 1/2 in and 1/4 in mesh to remove large plant matter and make flowable, then air-screen. (No awns or appendages to remove.)
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Central Iowa Germplasm (IA Zone 2), Northern Iowa Germplasm (IA Zone 1), Southern Iowa Germplasm (IA Zone 3)
Cultivated variety (cultivar): Midas (KS)
- References
Chayka, K. (n.d.). Heliopsis helianthoides (smooth oxeye). Minnesota Wildflowers. https://minnesotawildflowers.info/flower/smooth-oxeye
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Ox-eye. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 88). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). False sunflower - Heliopsis helianthoides. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/fs_sunflowerx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 36–37). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Ox-eye. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 162–163). University of Iowa Press.
Smith, A. R. (2020, November 6). Heliopsis helianthoides (Linnaeus) Sweet. Flora of North America. http://floranorthamerica.org/Heliopsis_helianthoides
USDA NRCS National Plant Data Team. (n.d.). Heliopsis helianthoides (L.) Sweet. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=HEHE5
Species Guide Updated 12/11/2024
spotted beebalm
spotted beebalm dickeye
Monarda punctata, L.
Alternate Common Names: dotted horsemint, spotted horsemint, dotted monarda, dotted beebalm
Family: mint family (Lamiaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Short-lived perennial, spreading by rhizomes to form clumps with a bushy appearance.
Height: 1-3 ft

- Leaves and stem
Leaves opposite, lance-shaped to narrowly lanceolate, 1-3 in long by 1/4 to 1/2 in wide, softly hairy; stems are brown to purple, 4-angled and branched sparingly, appearing frosted due to their coat of short, dense hairs.
- Flower, fruit and seedhead
Flower: Whorls of flowers form an interrupted spike at the end of branching stems, with showy, white to lavender-pink, leaf-like bracts at the base of each whorl that persist even after the flowers themselves wither; flowers are up to 1 in long, yellow with purple spots, tubular in shape with a distinct upper and lower lip.
Fruit/seed head: Calyx tubes (fused sepals of the flowers) persist as whorls around stem nodes after leaves drop; multiple whorls of seedheads per stem help distinguish this species from its congener M. fistulosa in seed; four nutlets develop within each calyx tube.
Pollination: Insects, especially solitary wasps.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 90,000 seeds/oz (IA NRCS)
1000 seed weight: 0.27g (Seed Information Database)
Description: “Seed unit” is a nutlet, oval in outline, smooth and brown, about 1-mm long.
Typical seed test
PLS: 90% (n = 2)
Purity: 92% (n = 2)
Germination: 20% (n = 1)
Dormancy: 83% (n = 2)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic sandy soil; full sun; sand and hill prairies, sandy savannas, sand dunes, sandy fields; Wetland Indicator Status is Obligate Upland (UPL) for the Midwest; very well drained soils are recommended for seed production.
Conservation status: Global- G5, secure; Ohio, Pennsylvania, and Vermont- S1, critically imperiled; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe)
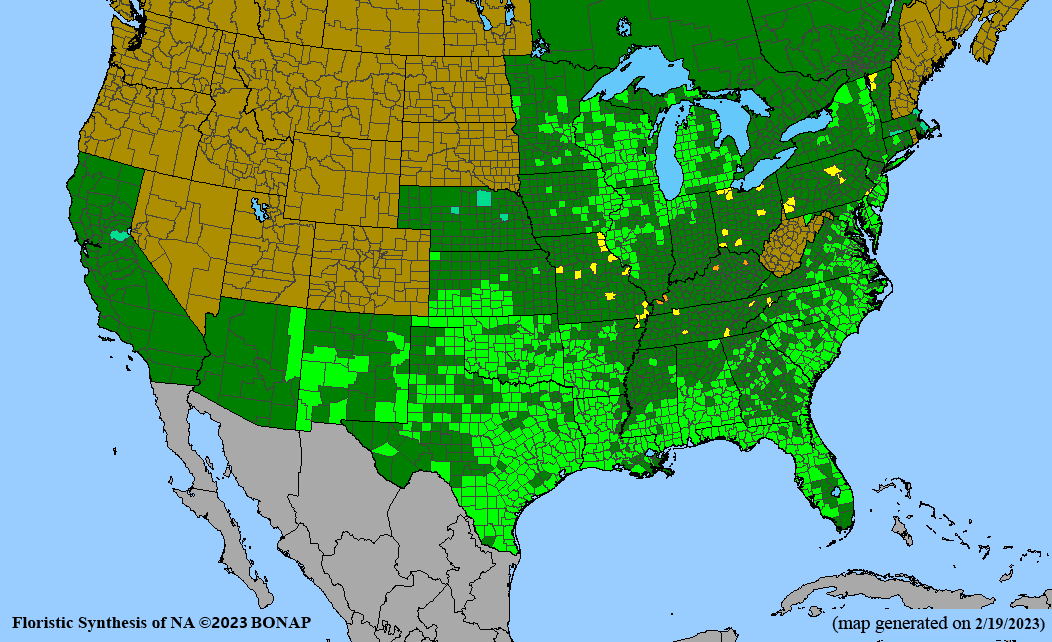
General Comments
Spotted beebalm is in the same genus as wild bergamot, M. fistulosa, but is found in habitats with sandy soils. Spotted beebalm differs from wild bergamot in having multiple whorls of flowers along a stem and white to lavender bracts beneath each flower cluster that are showier than the flowers themselves. Spotted beebalm has a long blooming season from mid to late summer, and remains showy after the flowers drop due to its persistent bracts. Its primary pollinators are solitary wasps such as great black wasps and golden digger wasps which drink nectar as adults but supply their young with insect prey. As wasps visit the flowers to sip nectar, the flowers’ stamens dust their thoraxes thickly with yellow pollen. When wasps are feeding at the flowers, they are not aggressive, and we have never been stung even when working among hundreds of plants (and their attendant wasps) in a plot. Spotted beebalm is short-lived in production plots but produces a lot of seed per plant and is relatively uncomplicated to manage, harvest, and clean.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Cold/moist stratification for 30 days may improve germination. Seed test reports suggest that a proportion of seeds may be dormant, though this is highly variable. Not using dormancy breaking procedures risks loss of dormancy-related genetic traits from production populations.
Sowing: Surface sow in the greenhouse about 2 months before the average date of last frost. Use caution when watering to avoid splashing seeds off the surface.
Transplanting: When plugs are well-rooted, move them outside to harden off, then transplant at 8-12 inch intervals into rows prepared with plastic mulch.
- Stand management
Weeds: Prepare clean, weed-free beds. Use plastic mulch to suppress weeds during the first growing season. Remove the mulch at the end of the season to allow plants to form clumps and to prevent moisture buildup. Mow or cultivate between rows and hand weed or rogue to prevent small-seeded weeds from contaminating the seed lot.
Pests: None noted.
Diseases: None noted.
- Seed production
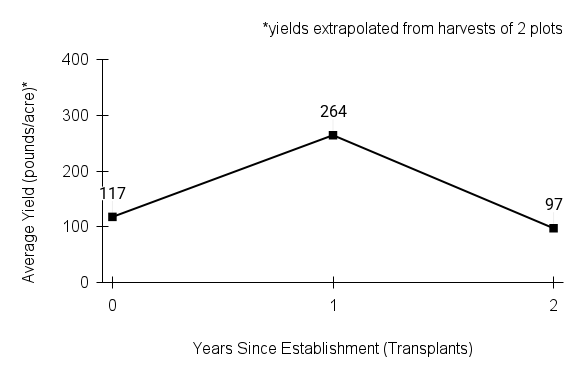 First harvest: Plants flower and set seed in the establishment year (from transplants), reaching peak harvest in the second year.
First harvest: Plants flower and set seed in the establishment year (from transplants), reaching peak harvest in the second year. Yield/acre: 120-260 pounds per acre (extrapolated from harvests of two plots)
Stand life: Plots are productive for two to three years. Subsequent yield declines are due to plant mortality in this short-lived species.
Flowering date: early July to late August in northeast Iowa
Seed maturity/Harvest date: mid September to mid October
Seed retention: Fairly low risk of shattering, except during high wind events.
Harvest date range at TPC (2017-2019): September 10 - October 18
Recommended harvest method: Hand clip early maturing plants, then combine when plot is at peak maturity.
- Seed cleaning and storage
Cleaning process: Brush to release seed remaining in calyx tubes, then airscreen.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 2 (central Iowa) and Zone 3 (southern Iowa)
- References
Chayka, K. (n.d.). Monarda punctata (spotted horsemint). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/spotted-horsemint
Christiansen, P., & Muller, M. (1999). Lamiaceae. An Illustrated Guide to Iowa Prairie Plants. (p.122). University of Iowa Press.
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Dotted horsemint. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 219). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Spotted bee balm - Monarda punctata. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/sp_balm.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Missouri Botanical Garden. (n.d.). Monarda punctata. Missouri Botanical Garden - Plant Finder https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=281405
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Monarda punctata L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=MOPU
Species Guide Updated 12/22/2025
spotted joe pye weed
spotted joe pye weed sagem
Eutrochium maculatum (L.) E. E. Lamont
Alternate Common Names: purple boneset, spotted trumpetweed
Scientific Synonyms: Eupatoriadelphus maculatus (L.) R. M. King & H. Rob., Eupatorium maculatum L., Eupatorium purpureum L. var. maculatum (L.) Darl., Eupatorium purpureum subsp. maculatum (L.) Á. Löve & D. Löve, Eupatorium trifoliatum var. maculatum (L.) Farwell
Family: aster or sunflower family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial, spreads slowly by rhizomes to form clumps.
Height: 2-10 ft (mostly 3-6 ft)

- Leaves and stem

Lance-shaped, up to 9 in long with serrated margins, whorled in groups of 4-5 (usually) at each node; unbranched stems purple to purple-spotted (the name ‘maculatum’ means spotted and refers to this trait).
- Flower, fruit and seedhead
Flower: 3-5 small, indistinct florets per head, in flat-topped to domed inflorescences with dozens to hundreds of pink to purplish heads (rarely white); inflorescences appear fuzzy due to the long styles that stick out of the florets.
Fruit/seedhead: Seed clusters ripen from the center outward, becoming tan and fluffy as seed matures; seed is wind-dispersed and susceptible to shattering in windy weather.
Pollination: Insects, particularly bees and butterflies.

- Seed
Seed characteristics
Seeds per ounce: 95,000 (IA NRCS)
1000 seed weight: 0.28 g (Seed Information Database)
Description: Slender, charcoal-gray seeds about 3 mm long with a tuft of tan pappus.
Typical seed test
PLS: 74% (n = 5)
Purity: 86% (n = 5)
Germination: 17% (n = 4)
Dormant: 72% (n = 4)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soil; full sun; wet prairies, sedge meadows, fens, ditches, and other wet places. The USDA classifies it as an Obligate Wetland species in the Midwest region. It benefits from irrigation in production systems.
Conservation status: Global- G5, secure; Idaho and West Virginia- S1, critically imperiled; Montana- S1/S2, critically imperiled to imperiled; Arizona, Virginia, and Georgia- S2, imperiled; Wyoming- S3, vulnerable (NatureServe)
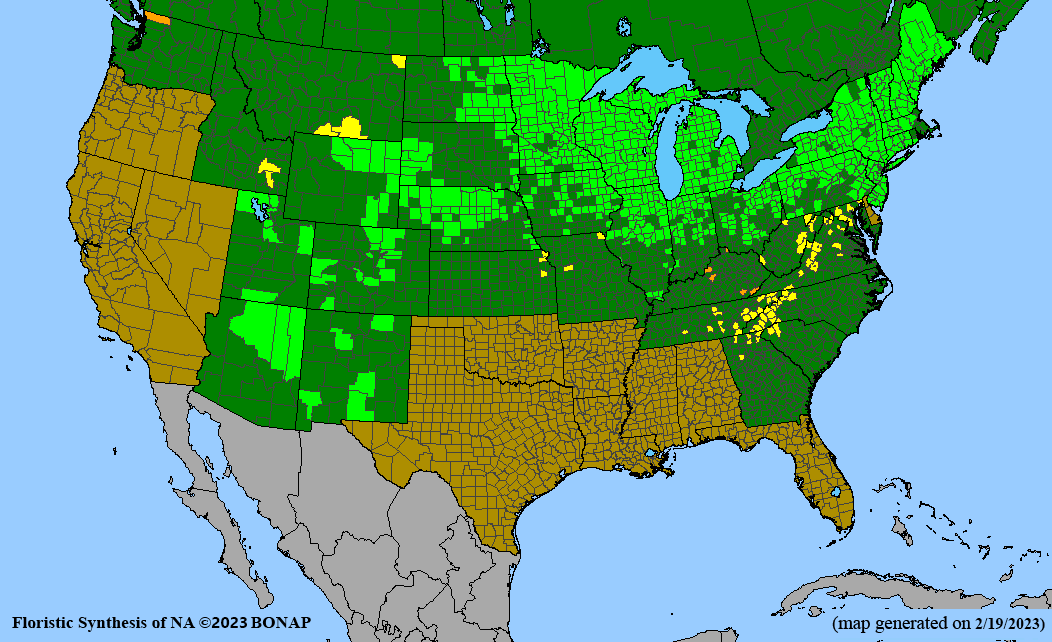
General Comments
The sweet-scented flowerheads attract numerous and diverse pollinators including the endangered rusty patched bumble bee. We once identified seven species of butterflies along a 150 foot row of flowering spotted joe pye weed in one 15-minute observation. Traditional uses of this species by Native tribes include treatments for digestive, urinary, kidney, and women’s complaints and using the hollow stems as straws. The clumped stems and whorled leaves produce dense shade that excludes most weeds from a well-established plot. Irrigation is important for seed production.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: 60 days cold-moist stratification.
Sowing: Seed is small and should be surface sown or very lightly covered or seedlings will not have enough energy to emerge. If started in germination flats, transplant to individual plugs when seedlings have their first pair of true leaves.
Transplanting: Seedlings are ready to transplant to the field about 8-12 weeks after starting in plugs, when their roots are well-branched and numerous root tips are visible at hole(s) in the base of the plug. Pop out a few plugs to check for adequate root development that will provide sturdy plugs for planting. A week or two before transplanting, move flats outside to ‘harden off.’ (See Propagation chapter in General Information for more details).
- Stand management
Weeds: In the first season after transplanting, weeds are suppressed by a plastic weed barrier. Plants spread by short rhizomes; in second and subsequent years, holes in plastic must be expanded or plastic removed to make room for new stems. Well-established plots shade out most weeds.
Pests: None noted.
Diseases: None noted.
Soil moisture: Irrigation is necessary in most soils to obtain maximum seed yield.
- Seed production
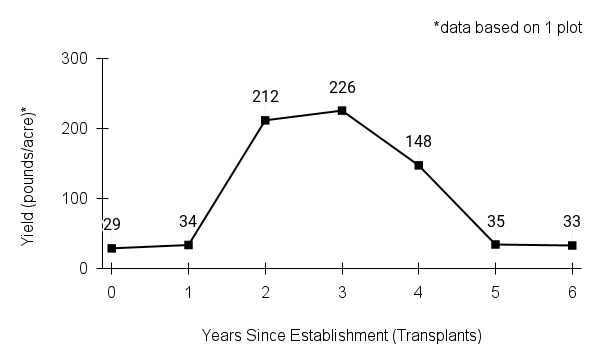 First harvest: Plants flower and set a little seed the first year when transplanted in spring.
First harvest: Plants flower and set a little seed the first year when transplanted in spring.Yield: 29-226 pounds/acre (based on 1 plot)
Stand life: Peak seed production in years 3-5, but plants are long-lived and vigorous for many years.
Flowering date: late July - early September in northeast Iowa
Seed maturity/Harvest date: second to third week of September
Seed retention: Seeds are wind dispersed soon after maturity, when fluff (pappus) expands in late August through September.
Harvest date range at TPC (2016-2022): Aug 26 - Oct 3
Recommended harvest method: Watch for the centers of seed clusters to begin shattering, and pick early maturing seed heads (clip stalks below seed clusters). If some heads in a cluster are still closed (not fluffy), pull apart a few heads to see if the seeds are dark colored and separate easily from the base (receptacle). Combine the rest of the plot at peak maturity. Turn off air or combine will disperse the fluffy seeds.
- Seed cleaning and storage
Cleaning process: If hand clipped, run dried material through a 1/4 in mesh to thresh seed from stalks. Use a brush machine (medium bristles, minimum vacuum) to remove pappus. Winnow with a box fan to separate seed from most of the pappus and chaff. Airscreen 2-3 times to finish cleaning. See Appendix C for specific settings.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1
Cultivated variety (cultivar): ‘Gateway’ is a compact cultivar used in landscaping.
- References
Chayka, K. (n.d.). Eutrochium maculatum (spotted joe-pye weed). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/spotted-joe-pye-weed
Eutrochium maculatum. (2024). Prairie Moon Nursery. https://www.prairiemoon.com/eutrochium-maculatum-joe-pye-weed-prairie-moon-nursery.html
Hilty, J. (n.d.). Spotted joe-pye weed - Eutrochium maculatum. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/sp_joepye.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Lamont, Eric E. (2020, November 6). Eutrochium maculatum (Linnaeus) E. E. Lamont. Flora of North America. http://floranorthamerica.org/Eutrochium_maculatum
Missouri Botanical Garden. (n.d.). Eutrochium maculatum “Gateway.” Plant Finder. https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=302488&is
Moerman, D. (2003). Native American ethnobotany database. BRIT. http://naeb.brit.org/
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
North Carolina State Extension (n.d.) Eutrochium maculatum. North Carolina Extension Gardener Plant Toolbox. https://plants.ces.ncsu.edu/plants/eutrochium-maculatum/
SER, INSR, RBGK, Seed Information Database (SID). (2023). Eupatorium maculatum. https://ser-sid.org/species/567dc915-c79f-4608-a9d6-e1351ee9a2cb
USDA NRCS National Plant Data Team. (n.d.). Eutrochium maculatum (L.) E.E. Lamont. USDA plants database. https://plants.usda.gov/plant-profile/EUMA9
Species Guide Updated 12/19/2024
stiff goldenrod
stiff goldenrod dickeye
Oligoneuron rigidum (L.) Small
Alternate Common Names: rigid goldenrod, hard-leaved goldenrod, prairie goldenrod, stiff-leaved goldenrod
Scientific Synonyms: Aster rigidus (L.) Kuntze, Solidago rigida* L.
*Solidago rigida is the accepted name in Minnesota and in the Flora of North America. The USDA Plants Database places this species in the genus Oligoneuron.
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from fibrous roots; several stems arise in a clump.
Height: 1-5 ft

- Leaves and stem
Leaves alternate, lower leaves long-stalked, upper leaves nearly clasping the stem, grayish green with short soft hairs; stem is finely hairy, unbranched.
- Flower, fruit and seedhead
Flower: Yellow flower heads, larger than typical for goldenrods, borne in a branched flat-domed cluster at top of stem.
Fruit/seedhead: Seed heads are fluffy due to a tuft of white pappus on each achene, seeds dispersed by wind.
Pollination: Insects including bees, butterflies, wasps, and beetles.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 41,000 (IA NRCS)
1000 seed weight: 0.94 g (Seed Information Database)
Description: Seeds are technically achenes, glabrous, bone-white, about 1/16 in long with long white plumes (pappus).
Typical seed test
PLS: 79% (n = 10)
Purity: 96% (n = 10)
Germination: 34% (n = 8)
Dormancy: 45% (n = 8)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry-mesic to wet-mesic soil; full sun; prairies, savannas, thickets, limestone glades, roadsides, railroads. Well-drained, loamy soils are preferred for seed production.
Conservation status: Global- G5, secure; District of Columbia- SX, presumably extirpated; Massachusetts- SH, possibly extirpated; Connecticut, Maryland, Pennsylvania, South Carolina, and West Virginia- S1, critically imperiled; New York and Virginia- S2, imperiled; Georgia and Wyoming- S3, vulnerable (NatureServe)
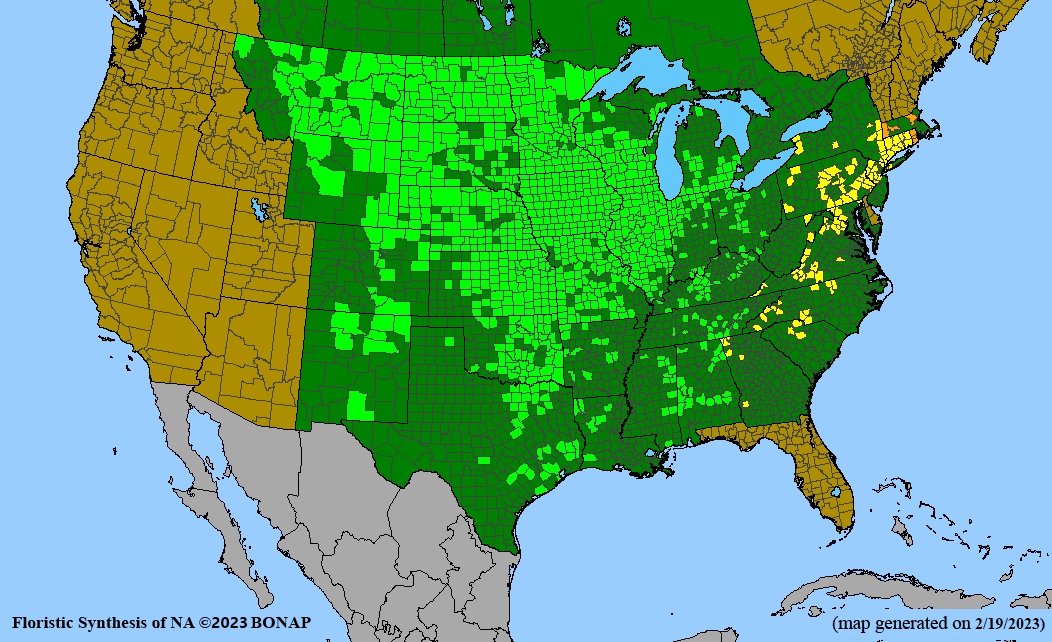
General Comments
Stiff goldenrod establishes readily from direct seed or transplants, and will spread from short rhizomes to form clumps. The flowers of this species are highly attractive to bees and other pollinators, including migrating monarch butterflies. The seeds are eaten by songbirds and gamebirds. Fields of this species can be combined but it is critical to harvest before plumes are dry and fluffy.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 1.0
Seeds per linear foot: 40
Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Wet stratify 8-12 weeks at 40˚ F.
Sowing: Sow seed in greenhouse two months before last frost free date.
Transplanting: Transplant into bare soil in rows convenient for tillage equipment, or into weed barrier at 8-12 in intervals after all danger of frost is past.
- Stand management
Weeds: Post emergence grass herbicide, tillage, hand roguing. If transplanted into weed barrier or plastic mulch, this provides some weed suppression.
Pests: None noted.
Diseases: Foliage may be affected by rust.
- Seed production
First harvest: Flowering and seed set at end of second growing season from either greenhouse grown transplants or direct seeded, well-managed stand.
Yield: 100-250 bulk pounds/acre
Stand life: Peak harvests in second to fifth growing season. Seed production declines in subsequent years.
Flowering date: mid-August - mid-September in northern Iowa
Seed maturity/Harvest date: October in northern Iowa
Seed retention: Seed is wind dispersed soon after drying of plumes (pappus).
Harvest date range at TPC (2003-2011): Oct 9 - 25
Recommended harvest method: Combine after seed maturity but before more than 10% of the seedheads have turned white and fluffy. Otherwise, combining will simply contribute to dispersal of the seed crop. Harvested material will have to be forced-air dried and turned carefully to prevent mold and decay.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping thru 1/2 in and 1/4 in mesh to remove large particles. Remove plumes (pappus) with a debearder or brush machine, then air-screen.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zone 1 (Northern Iowa), Zone 2 (Central Iowa),and Zone 3 (Southern Iowa)
- References
Chayka, K. (n.d.). Solidago rigida (stiff goldenrod). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/stiff-goldenrod
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Stiff goldenrod. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 118). University of Wisconsin-Madison Arboretum.
Flora of North America. Solidago rigida Linnaeus. (n.d.). http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=242417297 .
Hilty, J. (2019). Stiff goldenrod - Oligoneuron rigidum. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/stf_goldenrodx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 42–43). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Stiff goldenrod. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 242–243). University of Iowa Press.
Semple, J. C., & Cook, R. E. (2020, November 6). Solidago rigida Linnaeus. Flora of North America. http://floranorthamerica.org/Solidago_rigida
USDA NRCS National Plant Data Team. (n.d.). Oligoneurin rigidum (L.) Small. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=OLRI
Species Guide Updated 12/4/2024
swamp lousewort
swamp lousewort dickeye
Pedicularis lanceolata Michx.
Alternate Common Name: swamp betony
Scientific Synonyms: Pedicularis auriculata Sm., Pedicularis pallida Nutt., Pedicularis virginica Poir.
Family: broomrape family (Orobanchaceae)
Functional Group: forbs (wildflowers), hemiparasites
Description
- Life cycle and growth form
Fibrous-rooted perennial, hemiparasitic, connects to the root systems of neighboring plants through structures called haustoria to obtain mineral nutrients.
Height: 1 - 3 ft

- Leaves and stem
Leaves up to 4 in long with coarsely textured surface, “fernlike” margins, opposite arrangement; sturdy stems are short-hairy to hairless and sparingly branched.
- Flower, fruit and seedhead
Flower: Cream colored flowers, about 1 in long, tubular and 2-lipped, with top lip that overhangs and curves over lower lip; arranged in dense spikes up to 4 in long.
Fruit/seedhead: A many-seeded capsule that splits open at maturity to release seeds.
Pollination: Primarily bumblebees; the flowers are twisted, and only larger-bodied bees that can learn to open the flowers are capable of accessing the pollen.

- Seed
Seed characteristics
Seeds per ounce: 44,000 (IA NRCS)
1000 seed weight: 0.57 g (measured at TPC using seed harvested from plots)
Description: Wrinkled, oval, brown seeds are winged along one side, approx. 1.5 by 2.5 mm with the wing; the shape is reminiscent of Chinese dumplings (pot stickers)
Typical seed test
PLS: 87.5%
Purity: 94.3%
Germination: 2%
Dormant: 90.8%
(averages obtained from 6 tests)
- Habitat and range
Habitat: Moist to wet soil; partial to full sun; wet sand prairies, fens, swamps, sandy ditches, shorelines; Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest.
Conservation status: Global- G5, secure; Kentucky- SX, presumably extirpated; Delaware- SH, possibly extirpated; Arkansas, Georgia, Maryland, Massachusetts, and North Carolina- S1, critically imperiled; Pennsylvania and Tennessee- S1/S2, critically imperiled to imperiled; Connecticut and West Virginia- S2, imperiled; New York- S2/S3, imperiled to vulnerable; Nebraska, New Jersey, and Virginia- S3, vulnerable (NatureServe)
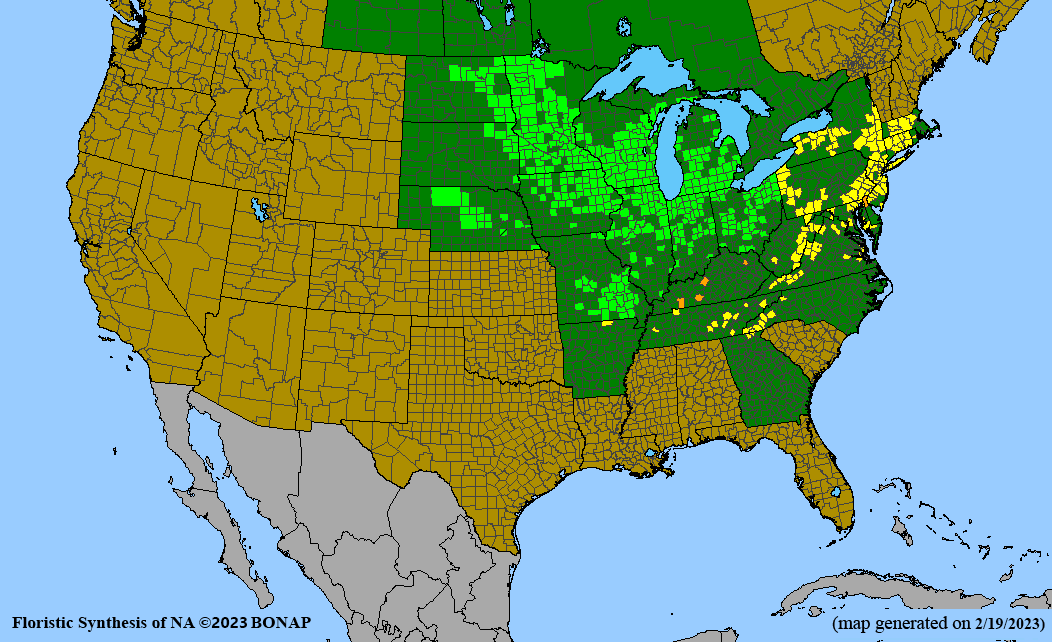
General Comments
Swamp lousewort is a hemiparasitic plant. This species is capable of photosynthesis but taps into the root systems of neighboring plants to obtain some of the mineral nutrients it needs for growth. Hemiparasitic plants may reduce the competitive dominance of their hosts, thereby promoting greater diversity in their plant communities. Swamp lousewort likely uses sedges, grasses, and composites as hosts in its wetland or wet prairie habitats. To establish plugs for seed production, we seeded stratified seed of swamp lousewort into plugs of two sedge species that could co-occur with it in nature. The seedlings transplanted well into irrigated production rows and produced abundant seed in the second year. The flowers are visited by worker bumble bees that can learn to twist open the flowers to access the pollen.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: Benefits from 60-day cold/moist stratification. Check seed frequently in later weeks of stratification period as some seeds may germinate in the cold.
Sowing: Start sedge host plants about one month before the lousewort stratification period is over. Sow two-three lousewort seeds into a shallow slit or divot made near the base of a host sedge in a plug. For this species we used trays of 50-cell plugs that are 4 in deep. As sedge host plants grow, trim them as often as necessary to keep light available to the lousewort seedlings. We found that trimming was needed more often with Carex bebbii hosts than with C. hystericina.
Transplanting: Transplant into prepared plasticulture beds with drip tape irrigation after danger of frost is past and plugs are sturdy with well-developed root systems. Move trays outside to “harden off” a week or more before transplanting.
- Stand management
Weeds: Plastic mulch suppresses weeds in the planting year, and dense growth of host sedges is competitive with many weeds. We mow between rows to further suppress weeds. Small seeded weeds such as amaranth and lambsquarters would be a concern for seed cleaning.
Pests: None noted.
Diseases: None noted.
Soil moisture: Irrigation is recommended. Drip tape can be installed at the same time the plastic mulch is laid.
- Seed production
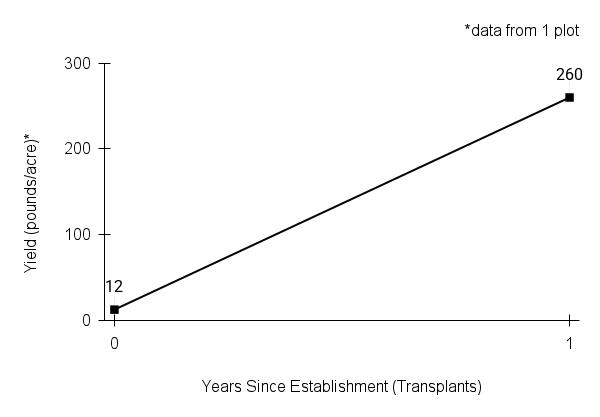
First harvest: A few plants flower and set seed in the planting year, but the first sizable harvest is in the year after transplanting.
Yield/acre: 260 lbs/acre (extrapolated from yield of one TPC production plot in the year after transplanting)
Stand life: Unknown at this time.
Flowering date: August - September in northeast Iowa
Seed maturity/Harvest date: mid-September to mid-October
Seed retention: Some seed is lost from open capsules, especially in high wind.
Harvest date range at TPC (2023-2024): September 7 - October 26
Recommended harvest method: We harvested the stems as the capsules matured, dried them, and passed them through the stationary combine. Combining in the field should also be effective, though some shattering may occur once capsules open.
- Seed cleaning and storage
Cleaning process: Seed that has been threshed through a combine may be passed through a coarse screen (1/4 in mesh) to remove remaining stemmy material, then airscreened. Hand collected material may need to be run through a brush machine to break up capsules and release seed.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI
Collection locations used in generating this ecotype are shown in the map below, overlaid on the Generalized Provisional Seed Transfer Zones of the US Forest Service.
- References
Chayka, K. (n.d.). Pedicularis lanceolata (swamp lousewort). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/swamp-lousewort
Dahlberg, L. (2022, February 3). Propagating Swamp Betony by Luke Dahlberg. Grassland Restoration Network. https://grasslandrestorationnetwork.org/2022/02/03/propagating-swamp-betony-by-luke-dahlberg/
Hilty, J. (2019). Swamp lousewort - Pedicularis lanceolata. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/sw_lousewort.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA-NRCS. (2022). Conservation cover native seeding calculator [Excel File]. Retrieved from https://bit.ly/IA_OTH_Conservation_Cover-Native_Seeding_Calculator_2022
USDA NRCS National Plant Data Team. (n.d.). Pedicularis lanceolata Michx. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=PELA2
Species Guide Updated 2/14/2025
swamp milkweed
swamp milkweed sagem
Asclepias incarnata L.
Alternate Common Names: rose milkweed, silkweed, water nerve root, white Indian hemp, swamp silkweed
Family: dogbane family (Apocynaceae), formerly assigned to the milkweed family (Asclepiadaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial, weakly rhizomatous, forming small clones of several stems; root systems are more fibrous than other commonly cultivated milkweeds such as butterfly milkweed (e.g., A. tuberosa).
Height: 2-5 ft

- Leaves and stem

Leaves 3-6 in long, usually narrowly lanceolate with smooth, untoothed edges and sessile on the stem or short-stalked, opposite arrangement; stems mostly hairless, usually unbranched (occasionally branched above).
- Flower, fruit and seedhead
Flower: Numerous, various shades of rose-pink with a pronounced fragrance similar to bubblegum, in domed clusters 2-3 in across; individual flowers five-parted, radially symmetrical, with a crown of five tubular hoods surrounding a central column, petals and sepals curved downward (typical milkweed flowers).
Fruit/seedhead: Pods (follicles) are 2-4 in long, smooth/waxy, and teardrop-shaped; follicles open along one side at maturity, revealing many shiny brown seeds each of which bears a flattened wing and a plume of soft, white floss.
Pollination: Pollination: Insects, particularly butterflies and bees.

- Seed
Seed characteristics
Seeds per ounce: 4,800 (IA NRCS)
1000 seed weight: 3.65 g (Seed Information Database)
Description: Dark brown, oval, surrounded by flattened ‘wing’ and tuft of soft hairs.
Typical seed test
PLS: 89% (n = 11)
Purity: 98% (n = 11)
Germination: 7% (n = 7)
Dormant: 58% (n = 7)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet, neutral to slightly acidic soil; partial to full sun open floodplains, lakeshores, ditches, wet prairies. Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest. Benefits from irrigation in production systems.
Conservation status: Global- G5, secure; Arizona, Mississippi, Montana, and Nevada- S1, critically imperiled; Arkansas, Idaho, and Louisiana- S2, imperiled; Wyoming- S2/S3 imperiled to vulnerable; Georgia- S3, vulnerable (NatureServe)
General Comments
Swamp milkweed is a valuable addition to pollinator habitat plantings on wetter soils and a showy, mannerly milkweed for home landscaping. This species is an important host for caterpillars of the monarch butterfly which feed upon its leaves. The fragrant flowers attract and provide nectar for numerous pollinators including various species of butterflies, bees, wasps, and flies. The strong, silky stem fibers are used as nesting material by songbirds and have traditionally been used for spinning and weaving by Native peoples, giving rise to some of the alternate common names such as silkweed and white Indian hemp. Establishment from plugs is rapid, and seed can be harvested the first fall, but production stands are short-lived (2-3 years).
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: To break dormancy, use 30-60 days cold stratification (check frequently for early germination) or a 24-hour soak in 250 ppm gibberellic acid (GA-3).
Sowing: Seed directly into plugs (2-3 seeds per cell) or on germination flat and lightly cover with potting mix, or germinate between sheets of moistened paper toweling and move to individual plugs as radicles (seed roots) emerge from seeds; start seeds 8-10 weeks before the average frost-free date.
Transplanting: Move plug flats outdoors 1-2 weeks before transplanting to allow seedlings to adjust to ambient conditions; plant with 1 ft spacing in plastic mulch with drip irrigation.
- Stand management
Weeds: Field preparation through repeated tillage and application of plastic mulch reduces weed issues; hand harvesting of pods as they mature results in very pure seed.
Pests: Non-native oleander aphids (Aphis nerii) can form dense infestations, reducing plant vigor and causing abortion of flowers or pods or, in extreme cases, death of the plant. Scout for these golden yellow aphids when plants are in bud, as it is easier to control small aphid infestations. Aphid predators (e.g., ladybug larvae, hoverfly larvae, and lacewing larvae) and aphid mummy wasps help reduce damage. Use the least toxic treatment possible (e.g., horticultural oils or insecticidal soaps) to avoid harming aphid enemies and pollinators, as aphids often occur at the time plants are in flower. Native seed-feeding milkweed bug species (Oncopeltus fasciatus and Lygaeus kalmii) pierce pods and feed on seeds. Manually remove clusters of red-orange milkweed bug nymphs from pods. A small proportion of pods is ruined by larvae of native milkweed stem weevils (Rhyssomatus lineaticollis or R. annectans). These appear as grubs or pupae inside the cluster of seeds. Affected pods have a small entry hole on the side and often mature prematurely. Earlier in the growing season, red milkweed longhorn beetles (Tetraopes spp.) feed on the tips of milkweed leaves. Their larvae feed within milkweed roots and rhizomes and may weaken plants over time. Monarch butterflies (Danaus plexippus) are attracted to lay eggs on swamp milkweed. In smaller-scale production systems, caterpillars can be hand-picked from production rows and transferred to nearby wild milkweeds before applying treatments for problem insects.
Diseases: Milkweeds are susceptible to a variety of fungal, bacterial, and viral diseases. Cultural methods such as reducing stand size or density, intercropping, and crop rotations may reduce disease spread and severity.
Hybridization risk: This species is known to hybridize with related species Asclepias hirtella, A. purpurascens, A. sullivantii, A, syriaca, A. tuberose, A. verticillata.
Soil moisture: Plant in naturally wet soils and/or supply irrigation, especially in dry weather.
Note: Refer to Milkweeds: A Conservation Practitioner’s Guide, published by the Xerces Society, for more detailed information on milkweed insects and diseases and their control.
- Seed production
 First harvest: Fall of planting year, when started from transplanted plugs.
First harvest: Fall of planting year, when started from transplanted plugs.Yield: 200-300 pounds/acre, with largest harvest in the first year (based on 4 plots)
Stand life: Two years from transplanting. Plants are reported to be longer lived in natural populations.
Flowering date: July in northern Iowa
Seed maturity/Harvest date: Early September - mid-October in northern Iowa
Seed retention: Seed is released as individual pods ripen and split open in early September through the beginning of October.
Harvest date range at TPC (2009-2020): Aug 31 - Oct 17
Recommended harvest method: Harvest by hand as pods (follicles) mature; collect pods that are changing color from green to yellowish and split when subjected to gentle pressure on the suture (seam), revealing dark brown seeds.
- Seed cleaning and storage
Cleaning process: Dry pods in cloth bags for two weeks with fan-forced air. Pass pods through a debearder or stationary combine to release seeds and detach fluff. Follow up by fan winnowing (outside on a relatively calm day) to remove most of the fluff. Air-screen the remaining material. Indent cylinder removes broken bits of pods and stems from seed.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source identified material: Natural Selections/Iowa Ecotype Zones 1, 2, IA
Cultivated variety (cultivar): ‘Ice Ballet,’ ‘Cinderella,’ and ‘Milkmaid’
- References
Borders, B. and E. Lee-Mäder. 2014. Milkweeds: A Conservation Practitioner’s Guide. 143 pp. Portland, OR. The Xerces Society for Invertebrate Conservation. https://www.xerces.org/sites/default/files/2018-05/17-031_02_XercesSoc_Milkweeds-Conservation-Guide_web.pdf
Chayka, K. (2010). Asclepias incarnata (swamp milkweed). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/swamp-milkweed
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Swamp milkweed. In Prairie plants of the University of Wisconsin, Madison Arboretum (3rd ed., p. 55). University of Wisconsin-Madison Arboretum.
Hilty, J. (2020). Swamp milkweed - Asclepias incarnata. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/sw_milkweed.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Kirk, S. and Belt, S. (2011). Plant fact sheet for swamp milkweed (Asclepias incarnata). USDA-Natural Resources Conservation Service, Norman A. Berg National Plant Materials Center. Beltsville, MD 20705. https://plants.usda.gov/DocumentLibrary/factsheet/pdf/fs_asin.pdf
Native Plant Trust (2024). Asclepias incarnata. https://plantfinder.nativeplanttrust.org/plant/Asclepias-incarnata
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Swamp milkweed. In Wildflowers of the tallgrass prairie: The upper Midwest (Second, p. 179). University of Iowa Press.
Schultz, Jan; Beyer, Patty; Williams, Julie. (2001). Propagation protocol for production of Container (plug) Asclepias incarnata L. plants USDA FS - Hiawatha National Forest Marquette, Michigan. In: Native Plant Network. US Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. https://NativePlantNetwork.org (accessed 2024/01/09).
Society for Ecological Restoration and Royal Botanic Gardens Kew. (2024). Asclepias incarnata L. SWAMP MILKWEED. Seed Information Database. https://ser-sid.org/species/62fbef4e-ed33-4c71-a18f-f78f9018fda7
University of Wisconsin. (2024). Common milkweed insects. Wisconsin Horticulture, Division of Extension. https://hort.extension.wisc.edu/articles/common-milkweed-insects/
Species Guide Updated 12/19/2024
swamp verbena
swamp verbena sagem
Verbena hastata L.
Alternate Common Names: blue vervain, simpler’s joy, American blue vervain, American simpler’s joy, wild hyssop
Family: verbena family (Verbenaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial, short-rhizomatous.
Height: 2-6 ft

- Leaves and stem

Leaves are opposite, lance-shaped (up to 6 in long and 1 in wide), coarsely toothed, strongly veined above, and usually short-hairy beneath; stems square, grooved, and green to purple, with flattened hairs, often branched in the upper half of the plant.
- Flower, fruit and seedhead
Flower: Individual flowers are blue-purple, 1/4 in across, with five expanded lobes (petals) attached to a short tube; flowers are arranged in a branched “candelabra” (panicle) of spikes; spikes elongate through the flowering season as new flowers emerge in whorls (rings) near the tops while seeds (nutlets) mature near their bases.
Fruit/seedhead: Each calyx of fused sepals contains four developing seeds (nutlets); spikes ripen from the bottom up.
Pollination: Insects, particularly bees.

- Seed
Seed characteristics
Seeds per ounce: 93,000 (IA NRCS)
1000 seed weight: 0.23 g (Seed Information Database)
Description: Seed unit is technically a type of dry fruit called a nutlet. Rust-brown nutlets are 2 mm long and approximately 0.5 mm wide.
Typical seed test
PLS: 97%
Purity: 100%
Germination: 13%
Dormant: 84%
(averages obtained from 5 tests of purchased seed lots)
- Habitat and range
Habitat: Swamp verbena grows best in full sun and moist to wet, organic-rich soils. Plants are typically found in wet places such as wet prairies, sedge meadows, fens, and ditches. Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest. Production fields benefit from irrigation.
Conservation status: Global- G5, secure; North Carolina- S2/S3, imperiled to vulnerable; Wyoming- S3, vulnerable (NatureServe)

General Comments
Swamp verbena is well suited for planting in moist soils or seasonally wet sites such as roadside ditches. It grows quickly and flowers in the planting year, producing abundant seed that is relatively easy to harvest and clean. Most mammalian herbivores avoid the bitter foliage, hence this species has persisted even in heavily grazed, wet prairie pastures. Swamp verbena has a long flowering period and is visited by diverse species of bees and small butterflies. The seeds are eaten by native sparrows and juncos. Swamp verbena has numerous uses in traditional Native medicine, including as a treatment for digestive and obstetric complaints. Caution: Extracts of this species are known to interfere with prescription medication and can cause vomiting and diarrhea in high doses.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Cold/moist stratification is recommended. Different sources suggest a minimum of 30 days (RNGR) up to 3 months (NRCS Plant Guide). Most northern Iowa accessions showed high rates of germination after 30 day stratification, but a few accessions appeared to have a higher degree of dormancy. In these cases, we saw a second flush of germination after a week of very high temperatures in the greenhouse, more than 2 months after sowing.
Sowing: Sow seed on surface of germination mix (light needed for germination).
Transplanting: Seedlings grow rapidly with fibrous root systems that form firm plugs for transplanting. Plan on sowing seed in greenhouse containers 8-10 weeks before transplanting. If seedlings grow too tall in plug trays, they should be pinched back to adjust the shoot:root ratio.
- Stand management
Weeds: Plastic mulch prevents weed competition in the first year, and tall fast-growing verbena plants are fairly competitive. Holes in the plastic may need to be widened to accommodate rhizomatous spread in the second and subsequent years. Focus weeding or roguing efforts on weeds that could contaminate the seed (i.e., species with small, elongated seed).
Pests: None noted. Bitter foliage deters mammalian herbivores.
Diseases: None noted, though plants appear to be short-lived (2-4 years) in production rows.
Hybridization risk: Maintain separation between fields of swamp verbena and other species in the genus Verbena (e.g., hoary vervain, Verbena stricta) as hybrids readily form.
Soil moisture: Irrigation is recommended.
- Seed production
 First harvest: In planting year, when grown from transplants.
First harvest: In planting year, when grown from transplants.Yield: 440-610 pounds/acre (based on 2 plots)
Stand life: Estimated 3-4 years, with peak harvest in year one.
Flowering date: late June - September
Seed maturity/Harvest date: mid-September - mid-October
Seed retention: Shattering begins as seedheads turn from purple to brown in mid-September through mid-October.
Harvest date range at TPC (2021-2023): Sept 16 - Oct 19
Recommended harvest method: Seed heads turn from green to purple to brown as they mature. Harvest when all, or nearly all, parts of the spikes have turned brown. Some seeds will shatter from lower parts of spikes as the seeds in the upper parts mature, but most seed is retained on the plant. Hand harvest early maturing individuals to preserve genetic diversity, then combine the rest of the plot at peak maturity.
- Seed cleaning and storage
Cleaning process
Hand-collected material: Dry on tarp or in a cloth bag for 2 weeks. Thresh by passing through a stationary combine or by stomping/beating material on the tarp or in a plastic tub. Pass through a ¼ in and ⅛ in mesh to remove sticks before airscreening.
Combined material: Hand-collected material: Dry on tarp or in a cloth bag for 2 weeks. Thresh by passing through a stationary combine or by stomping/beating material on the tarp or in a plastic tub. Pass through a 1/4 in and 1/8 in mesh to remove sticks before airscreening.
Note: Airscreening one-two times results in a very pure product.
Seed storage
cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI

- References
Chayka, K. (n.d.). Verbena hastata (blue vervain). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/blue-vervain
Hilty, J. (2019). Blue vervain - Verbena hastata. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/bl_vervain.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Kirk, S. and S.Belt. (2010). Plant fact sheet for blue vervain (Verbena hastata). USDA-Natural Resources Conservation Service, Norman A. Berg National Plant Materials Center. Beltsville, MD 20705.
Moerman, D. (2003). Native American ethnobotany database. BRIT. http://naeb.brit.org/
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
North Carolina State Extension (n.d.) Verbena hastata. North Carolina Extension Gardener Plant Toolbox. https://plants.ces.ncsu.edu/plants/verbena-hastata/
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
USDA-NRCS. (2022). Conservation cover native seeding calculator [Excel File]. Retrieved from https://bit.ly/IA_OTH_Conservation_Cover-Native_Seeding_Calculator_2022
USDA NRCS National Plant Data Team. (n.d.). Verbena hastata L. USDA plants database. https://plants.usda.gov/plant-profile/VEHA2
Species Guide Updated 12/18/2024
sweet coneflower
sweet coneflower dickeye
Rudbeckia subtomentosa, Pursh
Alternate Common Names: sweet black-eyed susan, fragrant coneflower
Family: aster and daisy family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with a fibrous root system, forming clumps of stems from short rhizomes.
Height: 3 - 6 ft
- Leaves and stem
Leaves alternate with short, dense hairs; lower leaves stalked and with 3-5 deep lobes, lobes and petioles decreased to absent from upper leaves; stem is hairy and grooved, branched above.
- Flower, fruit and seedhead
Flower: Composite heads 2-3 in across have 10 to 20 widely spreading, yellow rays surrounding a dark brown to black cone of numerous tiny, fertile disk florets.
Fruit/seedhead: Ray florets drop, leaving a cone of disk florets that ripen to form the “seeds” (achenes).
Pollination: Insects, especially native bees, but also wasps, flower flies, beetles and small to medium-sized butterflies such as skippers.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 43,000 (IA NRCS)
1000 seed weight: 0.94g (Seed Information Database)
Description: “Seed” is a blackish achene, about 3 mm long, wedge-shaped, with no pappus.
Typical seed test
PLS: 92% (n = 10)
Purity: 95% (n = 9)
Germination: 50% (n = 6)
Dormancy: 36% (n = 7)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to wet soil; partial to full sun; prairies, woodland openings and edges, savannas, streambanks; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; mesic loamy soils are recommended for seed production.
Conservation status: Global- G5, secure; Michigan- SX, presumed extirpated; Kentucky, Mississippi, and Texas- S1, critically imperiled; Tennessee- S2, imperiled; Iowa and Kansas- S3, vulnerable; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).

General Comments
Sweet coneflower (Rudbeckia subtomentosa) is less commonly seen in planted prairies than a related species, blackeyed Susan (R. hirta). Both species have been adopted into the horticultural trade due to their long-lasting and showy flower heads. R. subtomentosa is typically a taller plant, has foliage with lobed leaves and shorter hairs, and is longer-lived than its cousin. The flowers of sweet blackeyed Susan provide pollen and nectar to a wide variety of native bees and other insects, and the seedheads release a sweet smell when crushed. The plants are relatively long-lived in production. They are sensitive to broad-leaf herbicide drift, but how much this affects seed production is unknown.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Cold/moist stratification for 30-60 days.
Sowing: Sow in a greenhouse 2-3 months before last frost. Surface sow or very lightly cover the small seeds with a fine seed starting mix.
Transplanting: When seedlings form well-rooted plugs, transplant at 8-12 in spacing in rows prepared with plastic mulch.
- Stand management
Weeds: Prepare a clean, weed-free bed. Plastic mulch suppresses weeds in the first year or two. Plants are tall, and once well-established, compete effectively with smaller annual weeds and cool-season grasses. Hand weed or rogue out weeds that could contaminate the seed lot.
Pests: No serious issues noted. Caterpillars of the gorgone checkerspot butterfly form gregarious feeding clusters and strip a few leaves, but this is more of a curiosity than a problem.
Diseases: None noted.
Herbicide susceptibility: Leaves show signs of herbicide injury (cupping and twisting) from exposure to drift from synthetic auxin herbicides.
- Seed production
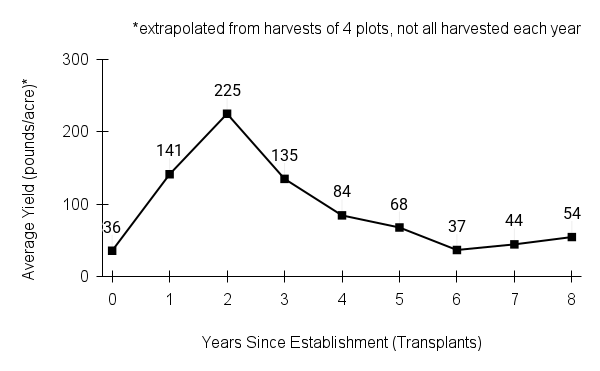 First harvest: A small harvest may be expected in the establishment year (from transplants).
First harvest: A small harvest may be expected in the establishment year (from transplants).Yield/acre: 40-225 pounds per acre (per acre yields extrapolated from harvest records of 4 plots)
Stand life: Plants persist for ten years or longer, although seed production declines after reaching a peak in the third year after transplanting.
Flowering date: early July through early September in northeast Iowa
Seed maturity/Harvest date: late September through mid October
Seed retention: Low risk of shattering; shattering begins in mid October.
Harvest date range at TPC (2004-2020): September 22 - November 3
Recommended harvest method: Combine
- Seed cleaning and storage
Cleaning process: Pass combined material through 1/2 in and 1/4 in mesh to remove larger particles, then airscreen. Several passes may be needed to separate the achenes from chaffy bracts of a similar size and weight. If harvested material contains unbroken heads (“cones”), brush with medium bristles to thresh achenes from heads.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone EA (eastern Iowa)
Cultivated varieties: Selections have been made for the horticultural trade.
- References
Chayka, K. (n.d.). Rudbeckia subtomentosa (Sweet Coneflower). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/sweet-coneflower
Christiansen, P., & Muller, M. (1999). Asteraceae. An Illustrated Guide to Iowa Prairie Plants. (p. 66). University of Iowa Press.
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Sweet coneflower. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 107). University of Wisconsin-Madison Arboretum.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Rudbeckia subtomentosa Pursh. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=RUSU
Species Guide Updated 12/29/2025
tall blazing star
tall blazing star dickeye
Liatris aspera Michx.
Alternate Common Names: rough blazing star, rough blazingstar, rough blazing-star, tall gay-feather, gayfeather, button snakeroot, rough gayfeather
Scientific Synonyms: Lacinaria scariosa var. intermedia Lunell, Liatris aspera var. intermedia (Lunell) Gaiser, Liatris aspera var. salutans (Lunell) Shinners, Liatris spheroidea var. salutans (Lunell) Shinners
Family: aster family (Asteraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial with a woody corm that can be divided.
Height: 1-4 ft

- Leaves and stem
Leaves narrowly lanceolate, alternate, with a prominent central vein and short stiff hairs; stem is rigid and rough with short hairs, green or purple in color, usually unbranched.
- Flower, fruit and seedhead
Flower: Pinkish purple heads, 1 in across, are spaced along the stalk, button-like, in a 6-18 in long spike; plants in production plots may be taller and produce robust, branched inflorescences. Heads of Liatris aspera are usually sessile or very short-stalked, compared with the stalked (pedunculate) heads of Liatris ligulistylis, Rocky Mountain blazing star, which is otherwise quite similar. Bracts on the underside of L. aspera heads are strongly cupped, while L. ligulistylis bracts tend to be flattened toward the top.
Fruit/seedhead: Dark brown seeds are 1/4 in long, ribbed, with a light brown pappus (fluff) that is finely barbed but not feathery; wind dispersed.
Pollination: Insects such as bees, butterflies, moths, and flies
- Seed
Seed characteristics
Seeds per ounce: 16,000 (IA NRCS)
1000 seed weight: 2.11 g (Seed Information Database)
Description: “Seeds” are achenes, nearly black, about 1/8 in to nearly 1/4 in long, with tufts of light brown hairs (pappus).
Typical seed test
PLS: 93% (n = 10)
Purity: 96% (n = 10)
Germination: 27% (n = 8)
Dormancy: 66% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to dry-mesic, even sandy or rocky soil; full sun; prairies, savannas, prairie remnants along railroads, upland forests, limestone glades. Upland, very well-drained, loamy soils are preferred for seed production. If soils are too dry or poor, seed production will be curtailed.
Conservation status: Global- G4, apparently secure; North Carolina- S1, critically imperiled; South Carolina- S2, imperiled; Georgia and Virginia- S3, vulnerable (NatureServe)
General Comments
This species is best propagated in the greenhouse, and transplanted in spring into a weed-free planting bed or weed barrier. Seedlings develop pea-size corms after two months in the greenhouse. Sometimes first year corms are exposed by frost-heaving over the winter, and may be eaten by voles. Species in the genus Liatris are known to hybridize, therefore proper isolation should be maintained between related species to avoid hybrid seed production (Levin 1968, Menhusen 1972). Liatris species are also produced commercially for the cut-flower industry and some species and cultivars have become popular in gardening and landscaping.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species
Greenhouse
Seed pre-treatment: Wet stratify 8-12 weeks at 40˚ F. Seed sometimes becomes moldy in stratification, and some growers add fungicide to the stratification media.
Sowing: Sow seed 1/4 in deep in the greenhouse two months before the last frost free date.
Transplanting: Harden off, transplant into bare soil in rows and mulch or transplant into a weed barrier at 8 in intervals after all danger of frost is past.
- Stand management
Weeds: Mow/cultivate between rows, mulch within rows. Post emergence grass herbicide, tillage, hoeing, hand roguing. Very sensitive to soil disturbance during bolting/flowering, so clip weeds rather than pulling or hoeing once flower stalks are apparent.
Pests: Voles will eat and/or cache corms, rabbits and deer eat young shoots, goldfinches consume seed as it ripens.
Diseases: Powdery mildew, root-knot nematodes, stem rot, verticillium wilt.
Hybridization risk: This species has been known to hybridize with related species Liatris acidota, L. ligulistylis, L. punctata, L. pycnostachya, and L. squarrosa.
- Seed production
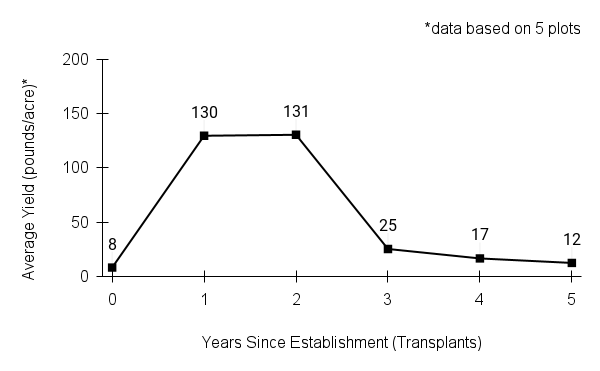
First harvest: Remains vegetative first year (seedlings), abundant flowering/seed production occurs second year. Fall corm division/transplanting results in abundant flowering the following growing season.
Yield: 8-130 bulk pounds/acre (extrapolated from harvests of 5 plots)
Stand life: Peak harvests second year. Good harvest third year if proper soils. Stand declines significantly fourth year and after. Plants tend to lodge second year when flowering.
Flowering date: early August - early September in northern Iowa
Seed maturity/Harvest date: late September - mid-October in northern Iowa
Seed retention: wind dispersed soon after maturity
Harvest date range at TPC (2003-2023): Sept 29 - Nov 6
Recommended harvest method: Combine at maturity, but before pappus is dry and fluffy. Seedheads mature from the top down along a stalk. When the topmost heads are fluffy, break open a few of the lower heads and observe for signs of maturity: dark-colored seeds that separate easily from the base of the head. Small plots may be hand harvested by clipping stalks as the seed matures, then drying the cut material in a building. Dry seed threshes easily from stalks.
- Seed cleaning and storage
Cleaning process: Pre-clean by scalping thru 1/2 in mesh to remove large particles and make the material flowable, brush gently with soft-bristles to remove ‘plumes’ (pappus), using care not to damage seed coat, then air screen.
Seed storage: cool/dry (33-50° F, 30-50% RH); stores well for a few years if seed is not damaged during cleaning.
Released Germplasm
Source Identified material: Northern Iowa Germplasm (IA Zone 1), Central Iowa Germplasm (IA Zone 2), Southern Iowa Germplasm (IA Zone 3)
- References
Chayka, K. (n.d.). Liatris aspera (rough blazing star). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/rough-blazing-star
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Rough blazing-star. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 95). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Rough blazingstar - Liatris aspera. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/rgh_blazingstarx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 38–39). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Levin, D.A. (1968). The Structure of a Polyspecies Hybrid Swarm in Liatris. Evolution, 22(2), 352-372. https://doi.org/10.1111/j.1558-5646.1968.tb05903.x
Menhusen, B.R. (1972). Ecology of the Prairie Species of the Genus Liatris. Third Midwest Prairie Conference Proceedings. Manhattan, Kan.: Division of Biology, Kansas State University. https://search.library.wisc.edu/digital/AL7JMUVRYYXDZO8S/pages/A56MVY3FXXELEL8L
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Nesom, G. L. (2020, November 5). Liatris aspera Michaux. Flora of North America. http://floranorthamerica.org/Liatris_aspera
Runkel, S. T., & Roosa, D. M. (2009). Rough blazing star. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 220–221). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Liatris aspera Michx. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=LIAS
Species Guide Updated 2/14/2024
white sagebrush
white sagebrush parkecag
Artemisia ludoviciana Nutt.
Alternate Common Names: white sage, prairie sage, western mugwort, Louisiana sage, prairie wormwood, cudweed, mugwort, dark-leaved mugwort, sagewort, western sage, sailor’s tobacco, sagebrush
Scientific Synonym: Artemisia vulgaris var. ludoviciana (Nuttall) Kuntze
Functional Group: forbs (wildflowers)
Family: aster or sunflower family (Asteraceae)
Description
- Life cycle and growth form
Perennial, spreading by rhizomes to form large colonies that exclude some other plants.
Height: 1-3 ft

- Leaves and stem

Alternate leaves, aromatic when crushed, of variable shape but mostly narrow, elongated ellipses up to 1 in wide and 3.5 (occasionally up to 5) in long, short-stalked or sessile, with silvery-white hairs on leaves and stems giving them a felt-like texture; stems may be branched or unbranched.
- Flower, fruit and seedhead
Flower: Individual florets are inconspicuous within silvery, barrel-shaped, 1/8 in heads arranged in clusters in upper leaf axils or in spike-like to open, branched arrays up to 17 in in length; at full flowering, yellow stamens and minute, yellow to reddish corollas may be visible; wind-pollinated.
Fruit/seedhead: Roughly cylindrical in shape, approximately 1/8 in long, pappus is absent, heads open to release seed (achenes) when mature.

- Seed
Seed characteristics
Seeds per ounce: 250,000 (IA NRCS)
1000 seed weight: 0.11 g (Seed Information Database)
Description: Cypsela (achene), elliptical in outline, about 0.5 mm long, light grayish-brown, without hairs or attached fluff (pappus).
Typical seed test
PLS: 84% (n = 11)
Purity: 92% (n = 11)
Germination: 30% (n = 10)
Dormant: 57% (n = 11)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic soil; full sun; sandy or rocky prairies, roadsides. Wetland Indicator Status is Obligate Upland (UPL) for the Midwest.
Conservation status: Global- G5, secure; Michigan- S1, critically imperiled (NatureServe)

General Comments
All above ground parts of the plant have a distinctive sage-like fragrance when rubbed or crushed. This species has traditional medicinal and ceremonial uses among numerous Native American tribes. Because it is wind-pollinated, white sagebrush is not considered a resource for pollinators, though it is a larval host for at least one species of moth caterpillar, Phaneta argenticostana. Its mode of vegetative spread produces a dense network of rhizomes and roots that function in erosion control.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience direct-seeding this species for seed production.
Greenhouse
Seed pre-treatment: 60 days cold-moist stratification (fine silica sand)
Sowing: Surface (seed is small and must not be buried too deeply); seed directly onto plug flats or start seedlings in germination trays and dibble into plugs when seedlings have first true leaves; start in greenhouse about 8-10 weeks prior to transplanting.
Transplanting: Harden off seedlings 1-2 weeks prior to transplanting; transplant with 12 in plant spacing in plasticulture plots or into bare soil in 36 in rows, after danger of frost; cut or remove plastic after the first full growing season to allow plants to spread by rhizomes.
Note: Also readily propagated through division or rhizome cuttings (see NRCS Plant Guide referenced below).
- Stand management
Weeds: Few issues as dense, young colonies tend to exclude weeds; other small-seeded members of the aster family (e.g., frost aster, Symphyotrichum pilosum, and marestail, Erigeron canadensis) could contaminate seed and should be rogued out before harvest.
Pests: None noted.
Diseases: None noted.
- Seed production
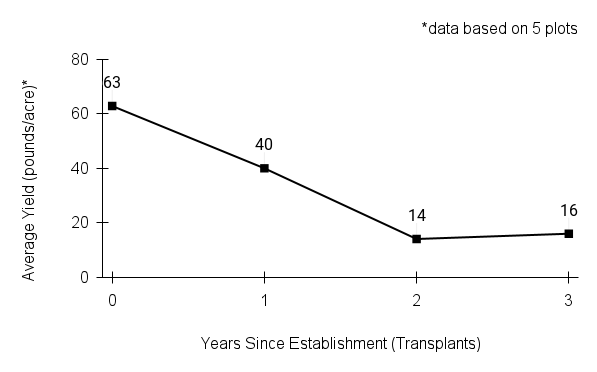 First harvest: In fall of first year when started from greenhouse transplants.
First harvest: In fall of first year when started from greenhouse transplants.Yield: 15-60 pounds/acre (based on 5 plots)
Stand life: Peak seed production in the first two years, then declining.
Flowering date: late August - September
Seed maturity/Harvest date: Mid-October in northeast Iowa; gauge maturity by sampling heads from several plants and crushing to reveal developing seeds (a hand lens is helpful); mature seed will have a grayish-brown color and separate easily from the receptacle; watch for heads to open and release seed when mature; seed shatters easily and will be lost if harvest delayed.
Seed retention: Shattering begins once seedheads open in mid to late October.
Harvest date range at TPC (2003-2023): July 17 - Oct 28
Recommended harvest method: Combine at maturity or cut/swath stems when about 10% of plants in the plot have open seed heads and lay to dry in shed, then run through stationary combine.
- Seed cleaning and storage
Cleaning process: Brush (Westrup LA-H) with stiff bristles and #14 screen mantle to release seed from heads, use minimal vacuum; airscreen several times.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source identified material: Natural Selections/Iowa Ecotype Project Zones 1, 2, and 3
Cultivated variety (cultivar): Summit (LA); horticultural varieties may also exist.
- References
Chayka, K. (n.d.). Artemisia ludoviciana (white sage). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/white-sage
Christiansen, P., & Muller, M. (1999). White sage - Artemisia ludoviciana Nutt. Prairie plants of Iowa - Artemisia ludoviciana Nutt. https://uipress.lib.uiowa.edu/ppi/display.php?record=Artemisia_ludoviciana
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). White sage. In Prairie plants of the University of Wisconsin, Madison Arboretum (3rd ed., p. 68). University of Wisconsin-Madison Arboretum.
Flora of North America Editorial Committee. 2006a. Flora of North America North of Mexico. Vol. 19. Magnoliophyta: Asteridae, part 6: Asteraceae, part 1. Oxford University Press, New York. xxiv + 579 pp.
Hilty, J. (2020). White sage - Artemisia ludoviciana. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/white_sagex.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 16, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Prairie sage. In Wildflowers of the tallgrass prairie: The upper Midwest (Second, p. 237). University of Iowa Press.
Shultz, Leila M. (2020, November 6). Artemisia ludoviciana Nuttall. Flora of North America. http://floranorthamerica.org/Artemisia_ludoviciana
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
Stevens, M., & Roberts, W. (2000). Plant guide - USDA Plants Database - White sage, Artemisia ludoviciana Nutt. https://plants.usda.gov/DocumentLibrary/plantguide/pdf/cs_arlu.pdf
Species Guide Updated 12/19/2024
whorled milkweed
whorled milkweed dickeye
Asclepias verticillata, L.
Alternate Common Name: eastern whorled milkweed
Family: dogbane family (Apocynaceae), formerly milkweed family (Asclepiadaceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial that forms extensive clonal patches from deep, spreading rhizomes.
Height: 1 - 2 ft
- Leaves and stem

Bright green needle-like leaves, up to 2-3 in long, with margins that are rolled to the underside, in whorls of 4-6 at nodes; slender stem, usually unbranched, with short hairs in vertical lines.
- Flower, fruit and seedhead
Flower: Typical milkweed flower, slightly greenish white, with a central reproductive column surrounded by a 5-part corona of nectar-filled hoods, 5 downcurved petals, and 5 sepals (hidden by the petals when in bloom); multiple 1-3 in rounded clusters of flowers (umbels) in upper leaf axils.
Fruit/seed head: Fruit is a slender, hairless, elongated follicle (commonly called a “pod”) up to 3-4 in long, releasing plumed oval brown seeds at maturity; seed is wind-dispersed.
Pollination: Insects, especially bees and wasps, as well as flies, butterflies, and moths. We have observed the endangered rusty patched bumble bee using these flowers.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 11,000 (IA NRCS)
1000 seed weight: 2.20g (Seed Information Database)
Description: Seeds are dark brown, teardrop-shaped with a flattened wing, about 5 mm long, with a tuft of silky fluff (coma or floss) prior to cleaning.
Typical seed test
PLS: 92% (n = 11)
Purity: 97% (n = 10)
Germination: 19% (n = 5)
Dormancy: 36% (n = 5)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic soil; partial to full sun; dry to dry-mesic prairies, woodlands openings, sandy savannas, limestone glades, rocky bluffs, fields, roadsides; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; moist to dry loamy to sandy soils are recommended for seed production.
Conservation status: Global- G5, secure; Delaware- SH, possibly extirpated; Rhode Island and Vermont- S1, critically imperiled; Massachusetts, New Jersey, New York, and Pennsylvania- S2, imperiled; Maryland and West Virginia- S3, vulnerable; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
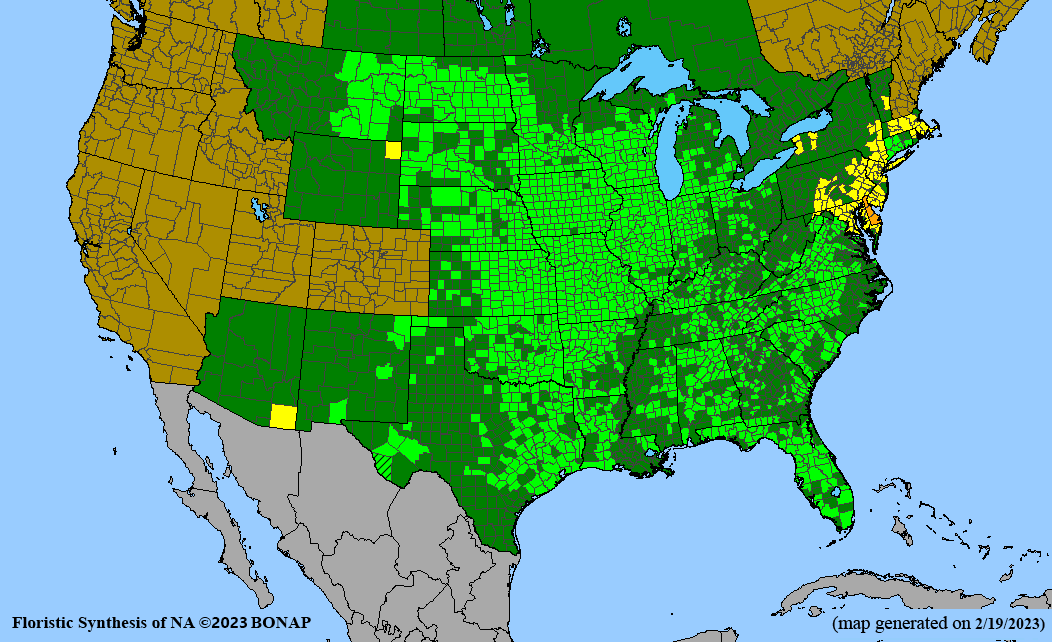
General Comments
In the late summer to early fall in the Tallgrass Prairie Center seed production fields, one of the best places to look for monarch butterfly caterpillars is on whorled milkweed plants. It may seem surprising to find large, late-instar caterpillars on these slender plants with their needle-like leaves, so unlike their robust cousin common milkweed. Each whorled milkweed stem is small, but the plant’s clonal nature means that an individual plant can have many stems, and a large caterpillar can simply mow down one after another. We often see clones of whorled milkweed on roadsides and highway medians that are mowed in spring, as well as in parts of native prairies where the vegetation is a bit sparse. In single-species, densely planted production plots, this species is productive for a year or two, then declines rapidly. We plan to investigate other methods, such as planting at low density within a grassy matrix, to see if the plants will remain productive for a longer period of time.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: 60 days cold/moist stratification or 24-hour treatment with 250 ppm GA-3 just prior to sowing.
Sowing: Sow seed, lightly covered, in the greenhouse about 2 months before the typical frost free date.
Transplanting: When plugs are well-rooted, move them outside to harden off, then transplant into prepared rows. We have planted them at 12 in spacing in plasticulture rows in the past, but the plants decline in year 2 and 3. We are planning to replant at a 3-4 ft spacing in an open, grassy matrix to allow vegetative spread.
- Stand management
Weeds: Prepare a clean, weed-free bed. Plastic mulch suppresses weeds in the first growing season but must be removed at the end of the year to allow new growth and vegetative spread. We will be attempting a new planting in an existing grassy area where the perennial grasses may help to suppress weeds. Since we hand collect the fruits as they ripen, weeds are of little concern for seed purity.
Pests: Invasive oleander aphids (bright yellow-orange) can form dense infestations on stems and flower heads. Native milkweed bug adults and their gregarious nymphs (young) pierce developing seed pods and damage the seeds. Monarch caterpillars and milkweed beetles feed on the foliage, but their density is rarely high enough to cause measurable damage.
Diseases: Plants in dense plantings appear unhealthy and stressed after the second year. We have not identified any particular diseases in these plots, but milkweeds are known to be susceptible to a variety of plant diseases.
Hybridization risk: This species may hybridize with other members of the milkweed genus, Asclepias. Maintain separation distances between plots of these species.
- Seed production
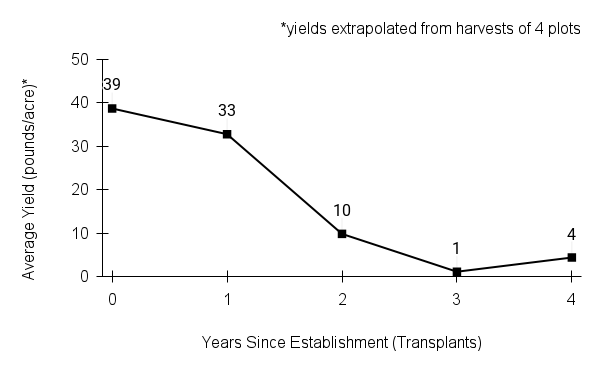 First harvest: Expect abundant flowering and seed set in the establishment year.
First harvest: Expect abundant flowering and seed set in the establishment year.Yield/acre: 30-40 pounds per acre during peak yield (extrapolated from harvests of four plots at TPC)
Stand life: Plants are most productive in the establishment year and their second year but decline rapidly thereafter.
Flowering date: late June through early September in northeast Iowa
Seed maturity/harvest date: Late August through early October in northeast Iowa; however, seed maturity may be delayed in the planting year or if plants are mowed in early summer.
Seed retention: High risk of shattering. Fluffy seed disperses from pods as they split open at maturity.
Harvest date range at TPC (2015-2023): August 29 - November 20, though usually completed by early October.
Recommended harvest method: Check fields several times per week during the harvest window and hand pick fruits when nearly mature but not yet open. The pods become lighter and yellower in color and soften as they ripen and pop easily when gently squeezed. Combining should be successful when about 10-20% of the pods in a plot have opened, though we have not attempted this.
- Seed cleaning and storage
Cleaning process: Protect eyes and airways from the abundant fluff that is released during the cleaning process. Large amounts of hand collected pods can be run through a debearder to break up the pods and remove the fluff from the seeds. Pass the material through 1/2 in and 1/4 in mesh to remove larger particles. If possible, do this step outside on a day with a light, steady wind. Finish by airscreening.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern Iowa), Zone 2 (central Iowa)
- References
Borders, B. & Lee-Mäder, E. (2014). Milkweeds, A Conservation Practitioners Guide. Xerces Society for Invertebrate Conservation. https://www.xerces.org/publications/guidelines/milkweeds-conservation-practitioners-guide
Chayka, K. (n.d.). Asclepias verticillata (whorled milkweed). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/whorled-milkweed
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Whorled milkweed. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 59). University of Wisconsin-Madison Arboretum.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Lady Bird Johnson Wildflower Center - The University of Texas at Austin. (2022). Asclepias verticillata. Plant Database. https://www.wildflower.org/plants/result.php?id_plant=asve
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
USDA NRCS National Plant Data Team. (n.d.). Asclepias verticillata L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ASVE
Species Guide Updated 12/23/2025
winged lythrum
winged lythrum dickeye
Lythrum alatum, Pursh
Alternate Common Names: winged loosestrife, wing-angled loosestrife, blue waxweed
Family: loosestrife family (Lythraceae)
Functional Group: forbs (wildflowers)
Description
- Life cycle and growth form
Perennial from a somewhat woody rootstock.
Height: 1 - 4 ft
- Leaves and stem
Leaves opposite on the lower half of the stem, becoming alternate in the inflorescence (upper stem); leaves hairless, stalkless, up to 3 1/2 in long and 1/2 in wide (smaller above), lanceolate to oval with rounded bases tapering to a pointed tip; stem is branched, hairless, 4-angled with the edges winged (expanded and flattened).
- Flower, fruit and seedhead
Flower: Flowers in axils of upper stem leaves in spikelike inflorescence up to 1 1/2 ft long; tubular flowers 1/2 in wide, usually 6-parted, lavender to pink; calyx is a persistent, narrow tube with six short, pointed lobes.
Fruit/seed head: Many-seeded, cylindrical capsule matures within the narrow calyx tube.
Pollination: Insects, especially long-tongued bees.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 3,000,000 (IA NRCS)
1000 seed weight: 0.05g (Seed Information Database)
Description: Seed is less than a millimeter long, narrow and light tan in color.
Typical seed test
PLS: 81% (n = 5)
Purity: 86% (n = 5)
Germination: 11% (n = 4)
Dormancy: 60% (n = 4)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soils; part shade to full sun; wet meadows or prairies, marshes, wet roadside ditches; Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest; irrigation is required for seed production.
Conservation status: Global- G5, secure; Connecticut, District of Columbia, Maryland, Massachusetts, Pennsylvania, Vermont, and Wyoming- S1, critically imperiled; Colorado, Virginia, and West Virginia- S2, imperiled; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
General Comments
Winged lythrum is a native species found in moist to wet prairies, sedge meadows, and wet ditches in Iowa. It should not be confused with purple loosestrife (L. salicaria), an aggressive introduced species that invades wetlands. The flowers of winged lythrum are less densely packed, it is generally a smaller, less conspicuous plant, and its stems have thin, flattened ridges on the edges (winged).
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: Cool/moist stratification for 60 to 90 days.
Sowing: Surface sow in the greenhouse about 2-3 months before the last frost. Use caution when watering to avoid splashing the seed out of the soil.
Transplanting: When seedlings form well-rooted plugs, move them outside to harden off, then transplant at 8-12 in spacing in an irrigated row with plastic mulch.
- Stand management
Weeds: Prepare a clean, weed-free bed prior to planting. Plastic mulch reduces weed pressure in the first year or two. A healthy stand of robust, well branched plants suppresses many weeds. Mow or cultivate between rows. Hand weed or rogue small seeded weeds that could contaminate the seed crop.
Pests: None noted.
Diseases: None noted.
- Seed production
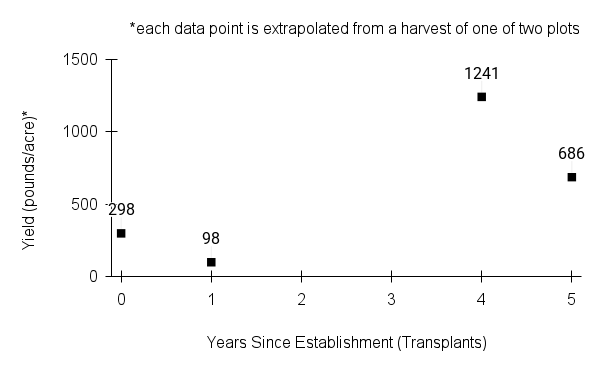 First harvest: Plants flower and set seed in the first year (from transplants).
First harvest: Plants flower and set seed in the first year (from transplants).Yield/acre: 100 - 1200 pounds per acre (This range is based on harvest records of 2 plots. The highest values were extrapolated from harvests of a very small plot. More reasonable estimates are probably in the 300-400 pound range.)
Stand life: Uncertain, but production in one small plot remained high into the 4th and 5th years after transplanting.
Flowering date: late June through September in northeast Iowa
Seed maturity/Harvest date: mid September through late October (later in establishment year)
Seed retention: Moderate risk of shattering. Seed is released from capsules as they open. Plants flower and set seed from the bottom up, so that some seed will be lost from capsules lower on the stem as seed is still maturing higher on the stem.
Harvest date range at TPC (2010-2025): September 10 - October 27
Recommended harvest method: Combine when most plants have more mature fruits than immature. Saving the stems that pass through the combine, drying them for a couple of weeks, and running them through the combine again can yield about 1/4 as much seed as the initial harvest. Check fields frequently in fall as the foliage turns red and senesces. Recall that fruits (capsules) mature within the narrow calyx tubes. Immature capsules are closed at the end and contain wet seeds, but mature capsules are open at the end, and the seed is dry and loose. Capsules closer to the bottom of the plant will be more mature at any point in time.
- Seed cleaning and storage
Cleaning process: Pass combined material through 1/2 in and 1/4 in mesh to remove larger stemmy material, then airscreen repeatedly. The seeds are small enough that some of them pass through the smallest sifting screen we have on hand. We save the sifting fraction and reclean it to keep small, filled seed in the seedlot.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone IOWA
- References
Chayka, K. (n.d.). Lythrum alatum (winged loosestife). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/winged-loosestrife
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Winged loosestrife. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 237). University of Wisconsin-Madison Arboretum.
Graham, S. A. (2022, May 9). Lythrum alatum Pursh. Flora of North America. http://floranorthamerica.org/Lythrum_alatum
Hilty, J. (2019). Winged loosestrife - Lythrum alatum. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/wng_loosestrife.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Lythrum alatum Pursh. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=LYAL4
Species Guide Updated 12/29/2025
Legumes
Legumes sagemThe Species Production Guides for legumes provide specific information about growing each of these species for seed production.
Scroll the list (alphabetized by scientific name) or press ctrl-f (or command-f) to search for any name in this page.
A printable file (pdf) is also provided on each species page for those needing a print version.
This section is a work in progress. We will continue to add new species guides as they are completed.
Amorpha canescens / leadplant
Baptisia bracteata / longbract wild indigo
Desmodium illinoense / Illinois ticktrefoil
Glycyrrhiza lepidota / American licorice
- Lespedeza capitata / roundhead lespedeza
Canadian milkvetch
Canadian milkvetch dickeye
Astragalus canadensis L.
Alternate Common Names: Canada milkvetch, Canada milk-vetch, milk-vetch, little rattlepod
Family: legume and pea family (Fabaceae (Leguminosae))
Functional Group: legumes
Description
- Life cycle and growth form
Perennial with a taproot, spreading by rhizomes.
Height: 1.5-3.5 ft
- Leaves and stem

Leaves alternate, odd-pinnately compound with 15 to 31 elliptical leaflets; stems are stiff, branched above, with some hairs, reddish when exposed to high sunlight.
- Flower, fruit and seedhead
Flower: Creamy greenish-white, narrow, elongated pea-like flowers, crowded in spikelike racemes 1.5 to 7 in long at tips of leafless stalks arising from leaf axils on upper portion of plant.
Fruit/seedhead: Spikelike clusters of erect, tough, dark brown pods, each 1/2 in long, with a sharp tip; pods split open from tips when mature to release seeds.
Pollination: Bumble bees and other long-tongued bees.
- Seed
Seed characteristics
Seeds per ounce: 17,000 (IA NRCS)
Seeds per pound: 275,000 (IA NRCS)
1000 seed weight: 1.97 g (Seed Information Database)
Description: Fruits are small pods containing several loose seeds. Pods are about 1 cm long (1/2 in), green at first, turning dark brown to black at maturity, splitting partially open. Seeds are a small, flat bean, about 2 mm (1/16 in) in diameter.
Typical seed test
PLS: 96% (n = 9)
Purity: 100% (n = 9)
Germination: 12% (n = 8)
Hard: 85% (n = 8)
(average of n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to wet-mesic soil; full sun; prairies, woodland edges, savannas, shorelines, abandoned fields. Wetland Indicator Status is Facultative (FAC) for the Midwest. Moist, fertile, loamy soils are preferred for seed production.
Conservation status: Global- G5, secure; District of Columbia- SX, presumed extirpated; Alabama, Georgia, Maryland, Pennsylvania- S1, critically imperiled; Michigan- S1/S2, critically imperiled to imperiled; Mississippi, Ohio, Utah, and Vermont- S2, imperiled; Colorado, North Carolina, Louisiana, and Nevada- S3, vulnerable (NatureServe)
General Comments
Canadian milkvetch is a short-lived species in seed production plots, usually dying out after a few years. It spreads prolifically from rhizomes the second year after establishment. It is usually found as small, somewhat stable colonies in prairies in disturbed areas, over a few years at least. Grazing or clipping prolongs the life-span of the plant, but of course this precludes seed production.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in 7 in rows and solid stands PLS lbs/acre: 2.1 6.3 Seeds/linear foot: 40
Seeding depth: 1/4-1/2 in
Seeding method: native seed drill
Seeding time: Dormant fall seeding of unscarified seed. Scarify and inoculate seed with Astragalus (Spec 1) inoculum for early spring planting.
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Scarify seed (see Seed Treatments) and wet stratify 10-14 days at 40° F.
Sowing: Sow seed in greenhouse 2 months before last frost free date.
Transplanting: Transplant mature seedlings into bare soil or weed barrier in rows convenient for tillage equipment after all danger of frost is past. Use a temporary weed barrier such as paper mulch or biodegradable plastic that breaks down or can be removed before the second growing season to accommodate plant spread from rhizomes.
- Stand management
Weeds: Mow stands above seedling height during establishment year. Use tillage and hand-roguing to control weeds.
Pests: Plots may need protection from rabbits and/or deer. Plants infested with black aphids become stunted and produce fewer flowers. Insect seed predators may become a problem.
Diseases: None noted.
- Seed production
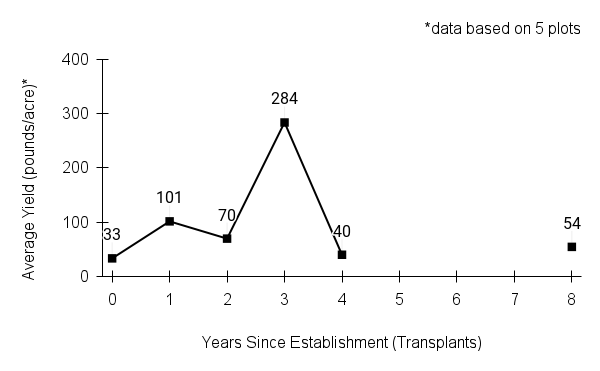
First harvest: Abundant flowering and seed set at end of second growing season from greenhouse grown transplants and well-managed direct seeded stands.
Yield: 30-280 bulk pounds/acre (averages based on 5 plots)
Stand life: Peak harvests in second to third years. Many stems die after flowering and setting seed, usually the second or third year after planting.
Flowering date: mid-July - early August in northern Iowa
Seed maturity/Harvest date: mid-August - early September in northern Iowa
Seed retention: Pods split partially open at maturity, and seeds will shake out of pods if disturbed by strong wind or passing animals.
Harvest date range at TPC (2003-2023): Aug 7 - Sept 25
Recommended harvest method: combine
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping thru 1/2 ft and 1/4 in mesh to remove large particles. If hand clipped, break up pods with beater bars in a brush machine. If combined then simply air-screen to clean (see appendix for settings).
Seed storage: cool/dry (33-50° F, 30-50% RH).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zones 1, 2, and 3
- References
Chayka, K. (n.d.). Astragalus canadensis (Canada milkvetch). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/canada-milkvetch
Hilty, J. (2019). Canada milkvetch - Astragalus canadensis. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/can_milkvetchx.htm
Houseal, G. A. (2007). Forbs legumes. In Tallgrass Prairie Center’s native seed production manual (pp. 56–57). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Astragalus canadensis L.. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ASCA11
Species Guide Updated 02/13/2025
longbract wild indigo
longbract wild indigo dickeye
Baptisia bracteata, Muhl. ex Elliott var. leucophaea (Nutt.) Kartesz & Gandhi
Alternate Common Names: Cream-colored false indigo, plains wild indigo, large-bracted wild indigo, long-bracted wild indigo, yellowish false indigo, cream wild indigo
Scientific Synonyms: Baptisia bracteata Muhl. ex Elliott var. glabrescens (Larisey) Isely, Baptisia leucophaea Nutt., Baptisia leucophaea Nutt. var. glabrescens Larisey
Family: legume and pea family (Fabaceae (Leguminosae))
Functional Group: legumes
Description
- Life cycle and growth form
Perennial growing from a taproot; spring shoots resemble asparagus but are fuzzy; in late fall, the plant breaks off near the ground and tumbles to disperse the seeds.
Height: 1-3 ft

- Leaves and stem
Leaves alternate, hairy, short-stalked or sessile on the stem, compound with 3 leaflets and two large, prominent stipules at the base of each leaf; stems hairy with wide-spreading branches.
- Flower, fruit and seedhead
Flower: Large, pale, creamy-yellow pea-shaped flowers in dense racemes that extend parallel to the ground or droop downward.
Fruit/seed head: Pods are inflated and green, turning black at maturity, 1-2 in long with pointed tips; seeds are small beans, golden-brown to olive in color
Pollination: Bumble bees and other large bodied bees.

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 1,400 seeds/oz (IA NRCS)
1000 seed weight: 11.51g (Seed Information Database)
Description: Seed is a small, plump bean about 5 mm in length, golden-brown and coated with a powdery, sticky resin.
Typical seed test
PLS: 93% (n = 6)
Purity: 100% (n = 6)
Germination: 7% (n = 4)
Dormancy: 68% (n = 4)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic soil; full sun; prairies, savannas, woodland openings; well drained soils preferable for seed production.
Conservation status: Global- G4, apparently secure; Minnesota- S3, vulnerable; (NatureServe)
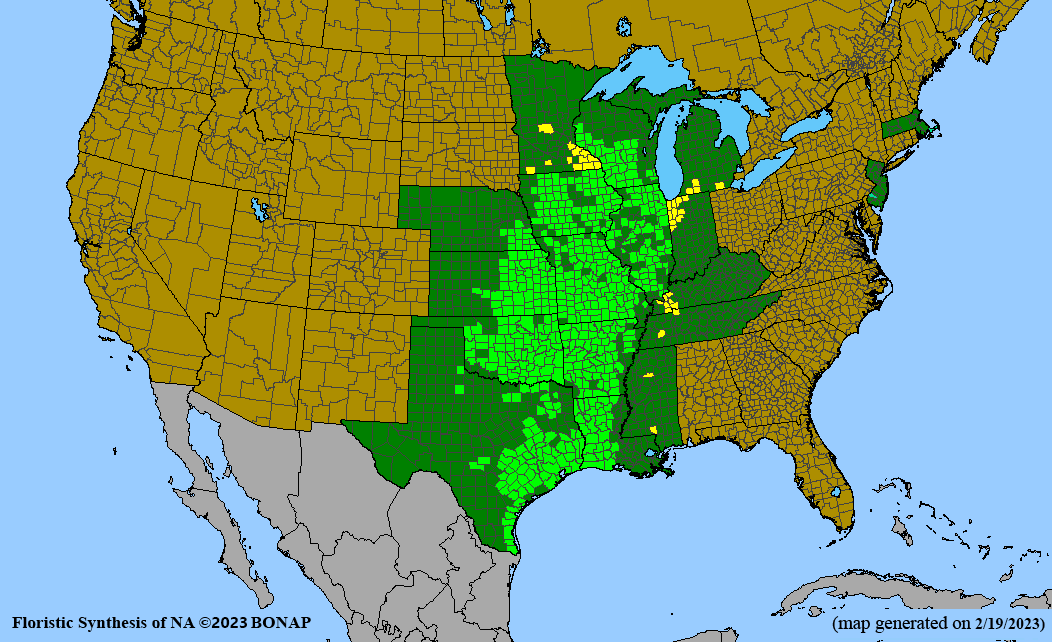
General Comments
The spring shoots of mature cream wild indigo plants come up quickly, resembling hairy asparagus, in late April in northeast Iowa. Blooms appear in May, making this large-flowered species an important source of food for new bumble bee queens. The pods blacken at maturity, and the entire plant turns charcoal-gray in fall. Pods eventually split open, revealing orderly rows of attached seeds, if they have not been devoured by the larvae of a host specific insect: the baptisia seed pod weevil. Seed yields are highly variable due to fluctuations in weevil populations. In natural populations, the stem breaks off at ground level in late fall and plants tumble with the wind, shaking out any seed remaining in the pods, aiding seed dispersal.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Scarify and stratify for 10-14 days in the refrigerator. Inoculate with Baptisia rhizobia if desired.
Sowing: Sow seed 1/4 in deep in plugs two months before average final frost. Damping off (fungal pathogen) can be a problem on seedlings if soil is kept too moist, or seedlings are planted too thickly. Add additional perlite to sterile potting mix to improve drainage. Avoid excess moisture on the soil surface by applying a thin layer of chick grit over the top of the soil, improving air circulation with fans, thinning seedlings, and/or watering from the bottom of the containers only. Seedlings form a fleshy taproot with few lateral roots, unless allowed to grow until taproot is air-pruned as it reaches the bottom drainage holes of the container. Plugs with vertical grooves and large bottom openings encourage air-pruning and branching of roots.
Transplanting: When seedlings are well-rooted plugs, transplant at 12 in spacing in rows mulched with plastic or other weed barrier.
- Stand management
Weeds: Adding a short, warm-season grass to production rows helps suppress weeds and provides fuel for prescribed fires.
Pests: A native, host-specific insect, the baptisia seed pod weevil, consumes nearly all seed in some years, making harvests of this species highly variable. Insecticide (permethrin) treatment may increase seed production slightly (15 more seeds per plant) (Horn and Hanula, 2004), but this must be weighed against potential damage to pollinators.
Diseases: None noted.
- Seed production
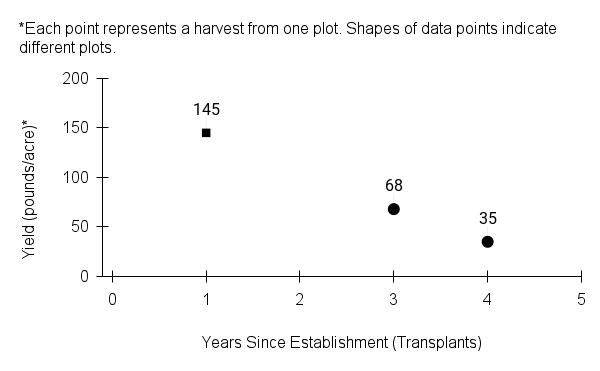 First harvest: Seed may be produced in the year following transplanting, however, yields are extremely variable depending on weevil populations. First measurable harvest in one field at TPC was in year 3.
First harvest: Seed may be produced in the year following transplanting, however, yields are extremely variable depending on weevil populations. First measurable harvest in one field at TPC was in year 3.Yield/acre: 35-145 pounds per acre (extrapolated based on harvest records of 2 plots. Note that each point on the yield graph represents a harvest from one plot. Plots were harvested only in years when weevil damage was light).
Stand life: Plants are very long-lived, persisting within a grassy matrix for 20 years or more, though they do not produce seed every year due to seed predation by weevils.
Flowering date: May in northeast Iowa
Seed maturity/harvest date: Aug - Sept
Seed retention: Not prone to shattering until late fall (October)
Harvest date range at TPC (2005-2024): Aug 4 - Oct 7
Recommended harvest method: Hand pick or combine
- Seed cleaning and storage
Cleaning process: Hand collected material may be stomped to break up pods. This step is not needed for combined material. Pre-clean air-dried material by scalping through 1/2 in mesh to remove large particles. Air-screen to clean (see Appendix C). Most black-colored seeds are non-viable and usually less dense than light-colored seeds, and most should be removed by increasing aspiration.
Seed storage: Cool/dry (33-50° F, 30-50% RH); seed may remain viable for 10 or more years
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zones 1, 2, 3
- References
Chayka, K. (n.d.). Baptisia bracteata (plains wild indigo). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/plains-wild-indigo
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Cream wild indigo. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 181). University of Wisconsin-Madison Arboretum.
Hilty, J. (n.d.). Cream wild indigo - Baptisia bracteata. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/cr_indigox.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Cream-colored false indigo. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 46–47). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Baptisia bracteata Muhl. ex Elliott. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=BABR2
Species Guide Updated 12/5/2025
purple prairie clover
purple prairie clover dickeye
Dalea purpurea Vent.
Alternate Common Names: violet prairie clover, thimbleweed, red tassel flower, purple prairie-clover
Scientific Synonym: Petalostemum purpureum (Vent.) Rybd.
Family: legume and pea family (Fabaceae (Leguminosae))
Functional Group: legumes
Description
- Life cycle and growth form
Perennial, with woody, branched taproot.
Height: 1-3 ft
- Leaves and stem
Leaves 1.5-3 in long, alternate, odd-pinnately compound with 3-7 very narrow leaflets, smooth but with black dots on lower leaf surface and a strong citrus odor when crushed; stems hairless to hairy and slightly ribbed when dry.
- Flower, fruit and seedhead
Flower: Individual flowers densely packed into a cylindrical spike about 1/2-3 in long; flowers open in whorls from the bottom to the top; tiny, 5-part flowers have purple petals and prominent, golden stamens.
Fruit/seedhead: Seed head is an elongate, compact head at the stem tip, composed of numerous dry, hairless pods which stay attached to the calyx until dislodged; strong scent when crushed.
Pollination: Insects, particularly bees, wasps, small butterflies, skippers, and beetles.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 18,000 (IA NRCS)
Seeds per pound: 288,000 (IA NRCS)
1000 seed weight: 3.19 g (Seed Information Database)
Description: Fruits are a 1-2 seeded legume. Seeds are small beans, about 2 mm (1/16 in) long, olive green to tan or brown.
Typical seed test
PLS: 97% (n = 11)
Purity: 100% (n = 11)
Germination: 83% (n = 10)
Hard: 14% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic, rocky or sandy soil; full sun; prairies, dunes, savannas. Very well-drained, loamy soils are preferred for seed production.
Conservation status: Global- G5, secure; Michigan and Ohio- SX, presumed extirpated; Georgia and Tennessee- S1, critically imperiled; Kentucky- S2, imperiled; Indiana- S3, vulnerable (NatureServe)
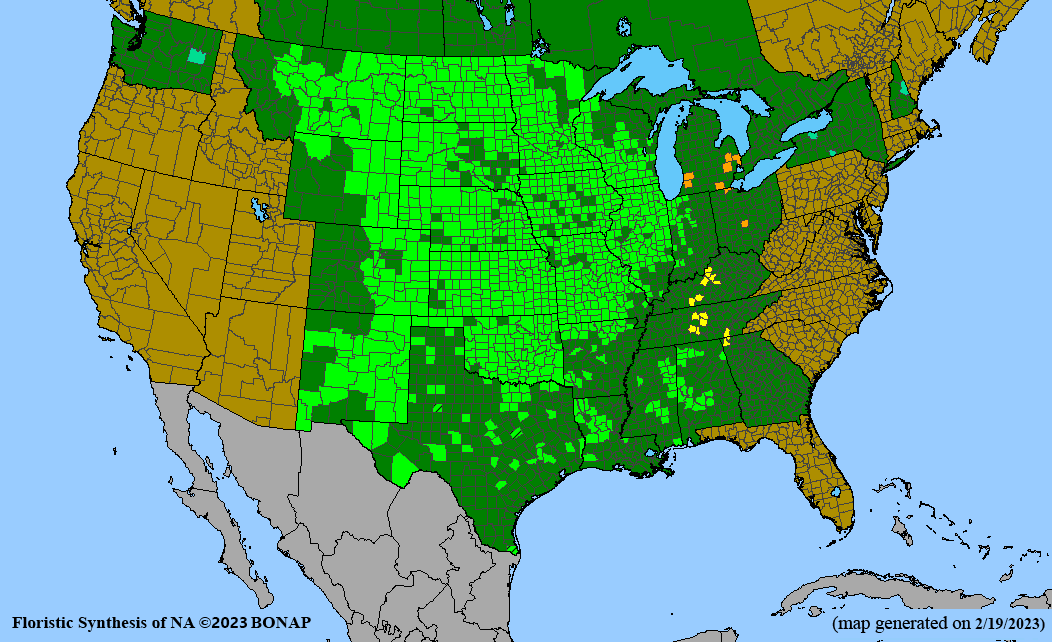
General Comments
Purple prairie clover is an important component of mesic to dry upland prairies. It tends to increase following spring burning (Bidwell 1990), though burning production fields is usually not an option because of a lack of continuous grass fuels to carry fire. Purple prairie clover seed should be dehulled when cleaned for the commercial market. Seed tests are more accurate on a dehulled seed, and seed count per pound is higher.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in 7 in rows or solid stand PLS lbs/acre: 2 6 Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: Dormant fall seeding of unscarified seed. Scarify and inoculate seed (Dalea, F inoculum) for spring planting.
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Scarify seed (see General Information: Propagation of Native Species). Moist stratification is not required, but seed should be stored in cold, dry conditions until planting time. A short stratification of 10-14 days may result in faster, more uniform germination.
Sowing: Sow seed 1/4 in deep in the greenhouse 2 months before the last frost free date. Damping off (fungal pathogen) can be a problem for seedlings if soil is kept too moist, or seedlings are planted too thickly. Avoid excess moisture on the soil surface by adding additional perlite to the sterile soil medium, applying a thin layer of chick grit (crushed quartzite or granite) over the top of the soil, improving air circulation with fans, thinning seedlings, and/or watering from the bottom of the containers only.
Transplanting: When root growth is sufficient to produce sturdy plugs, transplant seedlings into bare soil in rows convenient for tillage equipment or into a weed barrier at 8-12 in intervals after all danger of frost is past.
- Stand management
Weeds: Mow stands above prairie clover seedling height during establishment year. Poast (sethoxydim) or Prowl (pendimethalin) herbicide after establishment can be used to control weedy grasses. Plateau is labeled for pre- and post-emergence application. Note: These herbicides may not be labeled for this species in your state, always check the label and follow recommendations.
Pests: Herbivory by rabbits or deer may be a problem. Voles can kill many plants within a plot by feeding on the roots. Weevils may infest seed heads, reducing seed yield.
Diseases: None noted under field conditions. Damping off can be serious in a greenhouse environment (see above).
- Seed production
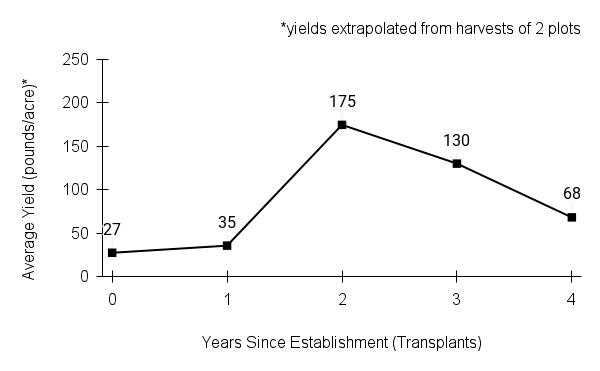 First harvest: Flowering and seed set occur at end of second growing season from greenhouse grown transplants and well-managed direct seeded stand.
First harvest: Flowering and seed set occur at end of second growing season from greenhouse grown transplants and well-managed direct seeded stand. Yield: 27-175 bulk pounds/acre (extrapolated from harvests of 2 plots)
Stand life: 5-10 years. Peak harvest third year.
Flowering date: July - early August in northern Iowa
Seed maturity/Harvest date: September in northern Iowa
Seed retention: Shattering potential is low. Seed heads hold seed into October.
Harvest date range at TPC (2004-2023): Sept 5 - Nov 5
Recommended harvest method: Combine. If plants still retain green leaves, do not cut any lower than necessary to harvest seed heads.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping through 1/2 in and 1/4 in mesh to remove large particles. Use a brush machine with stiff bristles to remove hulls, then air-screen. Re-brush any seed still in the hull, if necessary, and air-screen.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zones 1, 2, and 3
Selected Germplasm: Bismarck Germplasm (SD), Cuero Germplasm (TX).
Cultivated variety (cultivars): Kaneb (KS)
- References
Chayka, K. (n.d.). Dalea purpurea (purple prairie clover). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/purple-prairie-clove
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Purple prairie-clover. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 183). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Purple prairie clover - Dalea purpurea. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/ppr_cloverx.htm
Houseal, G. A. (2007). Forbs wildflowers. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 60–61). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Purple prairie clover - White prairie clover. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 176–177). University of Iowa Press.
USDA-NRCS. (n.d.). Conservation plant releases. Natural Resources Conservation Service. https://www.nrcs.usda.gov/plant-materials/cp/releases
USDA NRCS National Plant Data Team. (n.d.). Dalea purpurea Vent. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=DAPU5
Species Guide Updated 12/3/2024
roundheaded lespedeza
roundheaded lespedeza dickeye
Lespedeza capitata Michx.
Alternate Common Names: rabbit foot, round-headed bush clover, round-headed bush-clover, dusty clover
Scientific Synonyms: Lespedeza bicknellii House, Lespedeza capitata Michx var. stenophylla Bissell & Fernald, Lespedeza capitata Michx. var. velutina (E.P. Bicknell) Fernald, Lespedeza capitata Michx. var. Vulgaris Torr. & A. Gray
Family: legume and pea family (Fabaceae (Leguminosae))
Functional Group: legumes
Description
- Life cycle and growth form
Perennial from a central taproot.
Height: 2-5 ft
- Leaves and stem
Leaves alternate and compound, divided into three leaflets with prominent mid-veins and silky hairs; stem is erect, densely covered in stiff white hairs, usually unbranched; may be multiple stems from the base.
- Flower, fruit and seedhead
Flower: Small cream to white petals with purple spots at the throat, clustered into greenish, rounded heads at stem tip and in upper leaf axils.
Fruit/seedhead: Brown seed heads last through winter though seed is shed in fall; fruit is a fuzzy one-seeded pod.
Pollination: bees

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 8,000 (IA NRCS)
1000 seed weight: 2.87 g (Seed Information Database)
Description: Fruits are a one-seeded legume, seeds are a small bean, 4-5 mm (about 3/16 in) long.
Typical seed test
PLS: 95% (n = 11)
Purity: 99% (n = 11)
Germination: 79% (n = 10)
Dormancy: 7% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic, sandy soil; full sun; prairies, loamy or sandy savannas, dunes, roadsides, along railroads. Wetland Indicator Status is Facultative Upland (FACU) for the Midwest. Very well-drained loamy soils are preferred for seed production.
Conservation status: Global- G5, secure; West Virginia- SH, possibly extirpated; South Dakota- S2, imperiled; Kentucky and Vermont- S3, vulnerable (NatureServe)
General Comments
Roundhead lespedeza is commonly encountered in remnant prairies and establishes reliably in prairie plantings. It has high wildlife value, providing forage for mammalian herbivores, seed for songbirds and gamebirds, and floral resources for pollinators.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in rows
PLS pounds/acre: 4.0
Seeding depth: 1/4-1/2 in
Seeding method: native seed drill
Seeding time: Dormant fall seeding of unscarified seed. Scarify and inoculate seed (EL inoculum) for early spring planting.
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Scarify seed (see Propagation of Native Species: Seed Treatments) and wet stratify 10 days to 2 weeks at 40˚ F.
Sowing: Sow inoculated seed in the greenhouse two months before the last frost free date. Damping off (fungal pathogen) can be a problem on seedlings if soil is kept too moist, or seedlings are planted too thickly. Avoid excess moisture on the soil surface by amending the sterile potting mix with additional perlite, applying a thin layer of chick grit over the top of the soil, improving air circulation with fans, thinning seedlings, and/or watering from the bottom of the containers only. Seedlings form a fleshy taproot with few lateral roots, unless allowed to grow until taproot is air-pruned as it reaches the bottom drainage holes of the container. Plug trays with vertical grooves and wide drainage holes encourage root pruning and plug development.
Transplanting: When seedlings have sufficient root growth to form robust plugs and danger of frost is past, transplant into bare soil in rows convenient for tillage equipment or into a weed barrier at 8-12 in intervals. We have also had success transplanting seedlings into an existing Indiangrass stand that was weakened by mowing.
- Stand management
Weeds: Mow stands above seedling height during establishment year. Poast (sethoxydim) herbicide can be used to control weedy grasses. Prowl (pendimethalin) after establishment for grass control. Plateau should NOT be used on this species. Always read and follow label directions. Roundhead lespedeza can be planted with a warm-season grass such as Indiangrass for weed suppression and to support prescribed fire.
Pests: Herbivory may be a problem.
Diseases: Damping off can be serious in a greenhouse environment (see above).
- Seed production
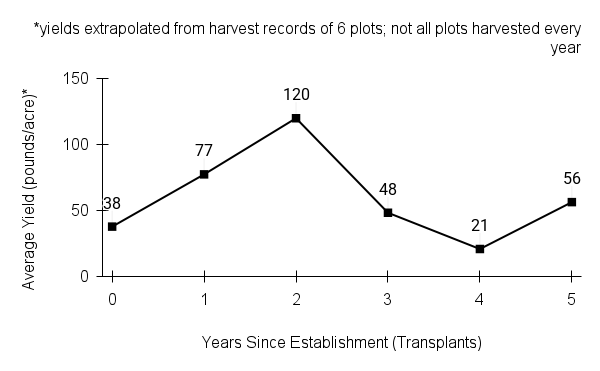 First harvest: Though some plants may flower the first year, two growing seasons are required for stand establishment and seed production. Peak production at TPC has been in the second and third years after planting.
First harvest: Though some plants may flower the first year, two growing seasons are required for stand establishment and seed production. Peak production at TPC has been in the second and third years after planting.Yield: 20-120 bulk pounds/acre (yields extrapolated from harvest records of 6 plots)
Stand life: 5-10 years; seed production typically decreases after 5 years.
Flowering date: mid-August - early September in northern Iowa
Seed maturity/Harvest date: October in northern Iowa
Seed retention: Shattering begins in late October into November
Harvest date range at TPC (2005-2023): Sept 26 - Oct 27
Recommended harvest method: combine
- Seed cleaning and storage
Cleaning process: Use brush machine/huller-scarifier to remove hulls, then air-screen (see Appendix C for settings).
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zone 1 (Northern Iowa), Zone 2 (Central Iowa), and Zone 3 (Southern Iowa)
Cultivated variety (cultivars): Kanoka (KS)
- References
Chayka, K. (n.d.). Lespedeza capitata (round-headed bush clover). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/round-headed-bush-clover
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014).Round-headed bush-clover. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 188). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Round-headed bush clover - Lespedeza capitata. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/rh_bushcloverx.htm
Houseal, G. A. (2007). Forbs legumes. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 64–65). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). Roundhead lespedeza. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 228–229). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Lespedeza capitata Michx. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=LECA8
Species Guide Updated 12/4/2025
showy ticktrefoil
showy ticktrefoil dickeye
Desmodium canadense (L.) DC.
Alternate Common Names: tick clover, Canadian tick trefoil, showy tick-trefoil, Canadian tick-trefoil, Canada ticklover
Scientific Synonym: Meibomia canadensis (L.) Kuntze
Family: legume and pea family (Fabaceae (Leguminosae))
Functional Group: legumes
Description
- Life cycle and growth form
Perennial from a woody taproot.
Height: 2-6 ft

- Leaves and stem
Leaves alternate, divided into three leaflets with rounded base and pointed tips, with sticky (hooked) hairs on undersides and narrow pointed bracts (stipules) on either side of the leaf petiole. Petiole is shorter than the stalk of the terminal leaflet (in contrast to Illinois ticktrefoil). Stem is usually unbranched, hairy.
- Flower, fruit and seedhead
Flower: Irregular, pea-shaped, 1/2 in long flowers, pink-purple with 2 yellow spots near the base of the upper lobe, arranged in spike-like racemes from stem tip and upper leaf axils.
Fruit/seedhead: Fruits are jointed pods called loments, 1-2.5 in long, covered in tiny hooked hairs to latch onto passing mammals, with 3-5 sections each containing one bean-like seed.
Pollination: bees
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 5,500 (IA NRCS)
Seeds per pound: 88,000 (IA NRCS)
1000 seed weight: 5.11 g (Seed Information Database)
Description: Seeds are small beans, about 2.5-3 mm (about 1/8 in), olive green to tan.
Typical seed test
PLS: 95% (n = 11)
Purity: 100% (n = 11)
Germination: 76% (n = 10)
Hard: 10% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry-mesic to wet-mesic soil; full sun; prairies, shorelines, woodland openings, roadsides, prairie remnants. Wetland Indicator Status is Facultative Upland (FACU) for the Midwest. Moist, fertile, well-drained loamy soils are preferred for seed production.
Conservation status: Global- G5, secure; Delaware and Maryland- SH, possibly extirpated; Virginia- S1, critically imperiled; Kansas- S3, vulnerable (NatureServe)
General Comments
Showy ticktrefoil is an important component of black soil prairies, increasing with spring burning. Its seeds are an important food source for upland game birds.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 30-36 in 7 in and solid stand PLS lbs/acre: 2.0 6.0 Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: Dormant fall seeding of unscarified seed. Scarify and inoculate seed for spring planting (Desmodium EL inoculum).
Weed Control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Scarify seed (see Propagation of Native Species: Seed Treatments). Moist stratification generally isn’t required, but seed should be stored in dry, cold conditions until sowing.
Sowing: Sow seed in greenhouse two months before last frost free date. Inoculate seed with appropriate rhizobium at time of sowing, if desired. Seedlings form a fleshy taproot with few lateral roots unless allowed to grow until taproot is air-pruned as it reaches the bottom drainage holes of the container. Plug flats with vertical grooves and wide drainage holes facilitate air-pruning.
Transplanting: When plants have sufficient root development to form sturdy plugs and danger of frost is past, transplant into bare soil in rows convenient for tillage equipment or into a weed barrier at 8-12 in intervals. Use care when transplanting to keep soil intact around the root system.
- Stand management
Weeds: For direct seedings, mow stand above showy ticktrefoil seedling height during establishment year to reduce weed competition and increase light to seedlings. Poast (sethoxydim) herbicide can be used for annual grass control, post emergence. Pursuit (imazethapry) can be used post-seeding for broadleaf weed control. Note: These herbicides may not be labeled for this species in your state, always check the label and follow recommendations.
Pests: Invasive Japanese beetles form feeding clusters on the inflorescences and in bad years can decimate flowering and seed production. For small scale production systems, a perimeter of beetle traps spaced about 5 m apart surrounding the plot can reduce damage. Traps are constructed of pheromone lures in funnels mounted atop 5-gallon buckets of soapy water. Seed weevils may infest and seriously curtail seed production. Aphids cause distortion of shoot tip growth and may inhibit flowering. Herbivory by deer, rabbits, and groundhogs may be an issue on young plants.
Diseases: Powdery mildew may affect foliage.
- Seed production
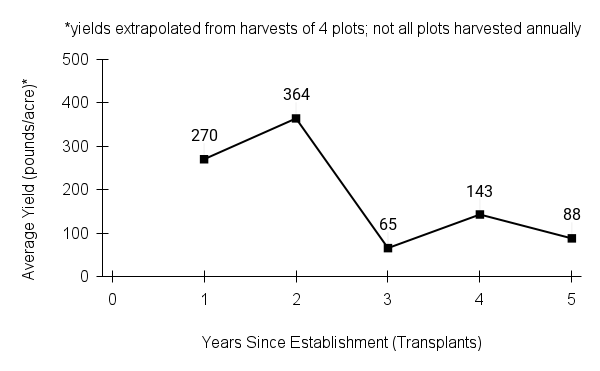 First harvest: Seedling growth is vigorous, and flowering and seed set may occur at end of first growing season from greenhouse grown transplants and well managed direct seeded stands.
First harvest: Seedling growth is vigorous, and flowering and seed set may occur at end of first growing season from greenhouse grown transplants and well managed direct seeded stands.Yield: 60-360 bulk pounds/acre (extrapolated from harvests of 4 plots; not all plots harvested annually)
Stand life: Stand may persist for 5 -10 years.
Flowering date: mid-July - mid-August in northern Iowa
Seed maturity/Harvest date: September in northern Iowa
Seed retention: Low risk of shattering; most seed loss begins late September into October
Harvest date range at TPC (2003-2021): Sept 1 - Oct 10
Recommended harvest method: Combine. Devise a system for collecting clumps of the sticky pods that don’t pass through the sieves and are ejected out the back of the combine.
- Seed cleaning and storage
Cleaning process: Use a brush machine to remove hulls (loments). Re-brush any seed still in the hull, if necessary. Airscreen to clean (see Appendix C for settings).
Seed storage: Cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zone 1 (northern Iowa), Zone 2 (central Iowa), and Zone 3 (southern Iowa)
Selected germplasm: Alexander Germplasm (IL).
- References
Chayka, K. (n.d.). Desmodium canadense (showy tick-trefoil). Minnesota Wildflowers. https://minnesotawildflowers.info/flower/showy-tick-trefoil
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Canadian tick-trefoil. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 184). University of Wisconsin-Madison Arboretum.
Great Plains Flora Association. (1991). Bean family. In T. M. Barkley, R. E. Brooks, & E. K. Schofield (Eds.), Flora of the Great Plains (2nd ed., p. 446). University Press of Kansas.
Hilty, J. (2019). Showy tick trefoil - Desmodium canadense. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/shw_trefoilx.htm
Houseal, G. A. (2007). Forbs legumes. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 62–63). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Desmodium canadense (L.) DC. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=DECA7
Species Guide Updated 12/4/2025
white wild indigo
white wild indigo dickeye
Baptisia alba (L.) Vent.
Alternate Common Names: white false indigo, large leaf wild indigo, large-leaved wild indigo, milky wild indigo, prairie false indigo
Scientific Synonyms: Baptisia lactea (Raf.) Thieret, Baptisia leucantha Torr. & A. Gray, Baptisia pendula Larisey var. macrophylla
Family: legume and pea family (Fabaceae (Leguminosae))
Functional Group: legumes
Description
- Life cycle and growth form
Perennial with a thick, deep taproot.
Height: 2-4 ft
- Leaves and stem
Leaves are alternate and compound with three smooth leaflets; stems are waxy and erect, multi-branched, and purplish; leaves and stems turn black after first frost.
- Flower, fruit and seedhead
Flower: Large, white, pea-like blossoms on erect racemes up to 2 ft long. Secondary racemes may also be present. Petals drop after pollination.
Fruit/seedhead: Inflated, cylindrical seed pods start out green but turn black when ripe, seeds are golden.
Pollination: Bumblebees
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 1,700 (IA NRCS)
Seeds per pound: 27,200 (IA NRCS)
1000 seed weight: 16.42 g (Seed Information Database)
Description: Seeds are a bean about 4-5 mm long (1/4 in), covered with a sticky resin when freshly mature.
Typical seed test
PLS: 95% (n = 11)
Purity: 100% (n = 11)
Germination: 9% (n = 9)
Hard: 88% (n = 10)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to wet-mesic soil; full sun; black soil or sand prairies, marsh and lake edges, thickets, limestone glades. Wetland Indicator Status is Facultative Upland (FACU) for the Midwest. Moist, well-drained soils are preferred for seed production.
Conservation status: Global- G5, secure; North Carolina- S2, imperiled (NatureServe)
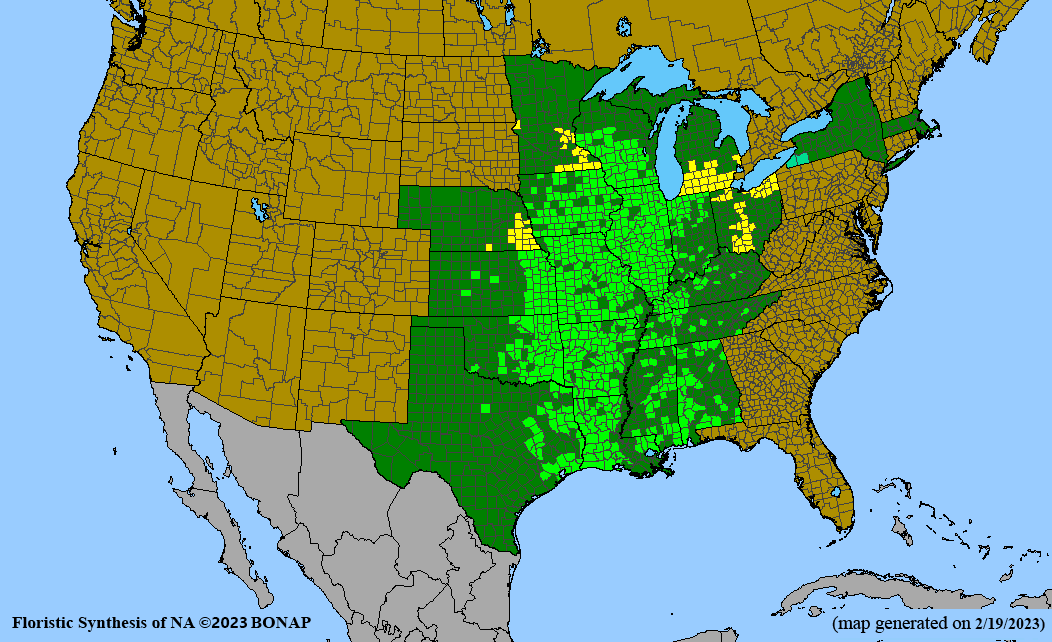
General Comments
The spring shoots of mature white wild indigo plants come up quickly, resembling asparagus, in late spring when soil temperatures warm. The tissues expand quickly, becoming shrub-like in form and blooming by early to mid June. Tissues turn black if bruised, as do seed pods at maturity. The entire plant blackens with fall dormancy. Pods eventually split open, revealing orderly rows of attached seeds, if they have not been devoured by the larvae of a host specific insect: the baptisia seed pod weevil. Seeds are somewhat sticky, initially. Stem breaks off at ground level in late fall and plants will tumble with the wind, shaking out any seed remaining in the pods, aiding seed dispersal.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species
Greenhouse
Seed pre-treatment: Scarify seed (see General Information: Propagation of Native Plants) and wet stratify 10 days to 2 weeks at 40° F. Inoculate seed with a Baptisia inoculum, if desired.
Sowing: Sow seed in greenhouse two months before last frost free date. Damping off (fungal pathogen) can be a problem on seedlings if soil is kept too moist, or seedlings are planted too thickly. Add additional perlite to sterile potting mix. Avoid excess moisture on the soil surface by applying a thin layer of chick grit over the top of the soil, improving air circulation with fans, thinning seedlings, and/or watering from the bottom of the containers only. Seedlings form a fleshy taproot with few lateral roots, unless allowed to grow until taproot is air-pruned as it reaches the bottom drainage holes of the container. Plugs with vertical grooves and large bottom openings encourage air-pruning and branching of roots.
Transplanting: Use care when transplanting to keep soil intact around the root system. Transplant healthy, well-rooted seedlings into bare soil in rows convenient for tillage equipment or into a weed barrier at 12 in intervals after all danger of frost is past.
- Stand management
Weeds: Mow stands above seedling height during establishment year. Control weeds with tillage and hand roguing. It may be desirable to overseed with a shorter growing warm season grass as a companion crop to reduce competition from weeds and to provide a fuel matrix for annual burning, which enhances seed production and seems to reduce seed predation by weevils.
Pests: Seed production can be curtailed or even eliminated in some years by a seed-eating weevil (Apion rostrum). The weevil oviposits eggs in the developing fruit, and the larvae emerge a few days later inside the sealed pods and feed on the developing seeds. Plants may also selectively abort pods containing fewer seeds due to seed predation. It may take a few years for weevils to find and colonize a new production field. Deer are known to eat the entire inflorescence while in bud.
Diseases: None noted.
Hybridization risk: Maintain separation between plots of Baptisia alba and other species in the genus: B. australis, B. bracteata.
- Seed production
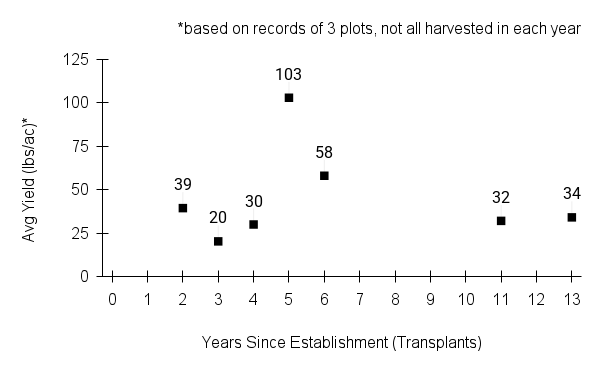 First Harvest: Plants are slow to reach reproductive maturity. Some flowering and seed set may occur in the third growing season under optimal conditions, but it may require 4 to 5 years for a full harvest.
First Harvest: Plants are slow to reach reproductive maturity. Some flowering and seed set may occur in the third growing season under optimal conditions, but it may require 4 to 5 years for a full harvest.Yield: 20-100 bulk pounds/acre (yields extrapolated based on harvest records from 3 plots)
Stand life: Plants appear to be long-lived, estimated stand life of at least 10 years.
Flowering date: mid-June - mid-July in northern Iowa
Seed maturity/Harvest date: September in northern Iowa.
Seed retention: Shattering occurs gradually through September into October.
Harvest date range at TPC (2004-2015): Aug 13 - Oct 21
Recommended harvest method: Combine or hand strip pods at maturity.
- Seed cleaning and storage
Cleaning process: Hand collected material may be stomped to break up pods. This step is not needed for combined material. Pre-clean air-dried material by scalping through 1/2 in mesh to remove large particles. Air-screen to clean (see Appendix C). Most black-colored seeds are non-viable and usually less dense than yellowish-colored seeds, and most should be removed by increasing aspiration.
Seed storage: Cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source identified material: Natural Selections/Iowa Ecotype Project Zones 1 (northern Iowa), 2 (central Iowa), and 3 (southern Iowa)
Cultivated variety (cultivars): Horticultural varieties may also exist.
- References
Chayka, K. (n.d.). Baptisia lactea (white wild indigo). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/white-wild-indigo
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). White wild indigo. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 180). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). White wild indigo - Baptisia alba macrophylla. Illinois Wildflowers. https://www.illinoiswildflowers.info/prairie/plantx/ww_indigox.htm
Houseal, G. A. (2007). Forbs legumes. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 58–59). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Baptisia alba (L.) Vent.. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=BAAL
Species Guide Updated 12/2/2024
Warm Season Grasses
Warm Season Grasses sagemThe Species Production Guides for warm season grasses provide specific information about growing each of these species for seed production.
Scroll the list (alphabetized by scientific name) or press ctrl-f (or command-f) to search for any name in this page.
A printable file (pdf) is also provided on each species page for those needing a print version.
This section is a work in progress. We will continue to add new species guides as they are completed.
Muhlenbergia racemosa / marsh muhly
Indiangrass
Indiangrass dickeye
Sorghastrum nutans (L.) Nash
Alternate Common Name: yellow Indiangrass
Scientific Synonyms: Andropogon nutans L., Sorghastrum avenaceum (Michx.) Nash
Family: grass family (Poaceae)
Functional Group: warm season grass
Description
- Life cycle and growth form
Perennial warm-season bunchgrass with short, scaly rhizomes.
Height: 3-7 ft
- Leaves and stem
Leaf blades up to 12 in long, constricted at the base, then widening to about 3/8 in, and tapered to a point, whitish midrib prominent near the leaf base; ligule with prominent pointed leaflike projections on either side which are sometimes referred to as the ‘mule-ears’, ‘boot straps’, or ‘gun-sight’ character of Indiangrass; stem is erect, hairless, and hollow.
- Flower, fruit and seedhead
Fruit/seedhead: Seedhead is a dense, golden-brown, plume-like panicle up to 1 ft long. Entire spikelets fall off when mature, leaving a bare stalk.
Pollination: wind

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 12,000 (IA NRCS)
1000 seed weight: 2.03 g (Seed Information Database)
Description: Seed unit is a fertile spikelet with a bent, twisted awn, about 1/2 in long, attached stalks (rachis and pedicel), hairy prior to debearding or brushing. Caryopsis smooth, brown, thickened, about 3-5 mm long.
Typical seed test
PLS: 87%
Purity: 92%
Germination: 22%
Dormant: 74%
(averages obtained from 12 tests of purchased seed lots)
- Habitat and range
Habitat: Dry-mesic to wet-mesic soil; full sun; prairies, grassy fens, scrubby barrens, savannas, roadsides, along railroads; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; deep, moist, well-drained soils preferred for seed production.
Conservation status: Global- G5, secure; Maine, Rhode Island, and Utah- S1, critically imperiled; Wyoming- S2, imperiled; Vermont- S3, vulnerable (NatureServe)
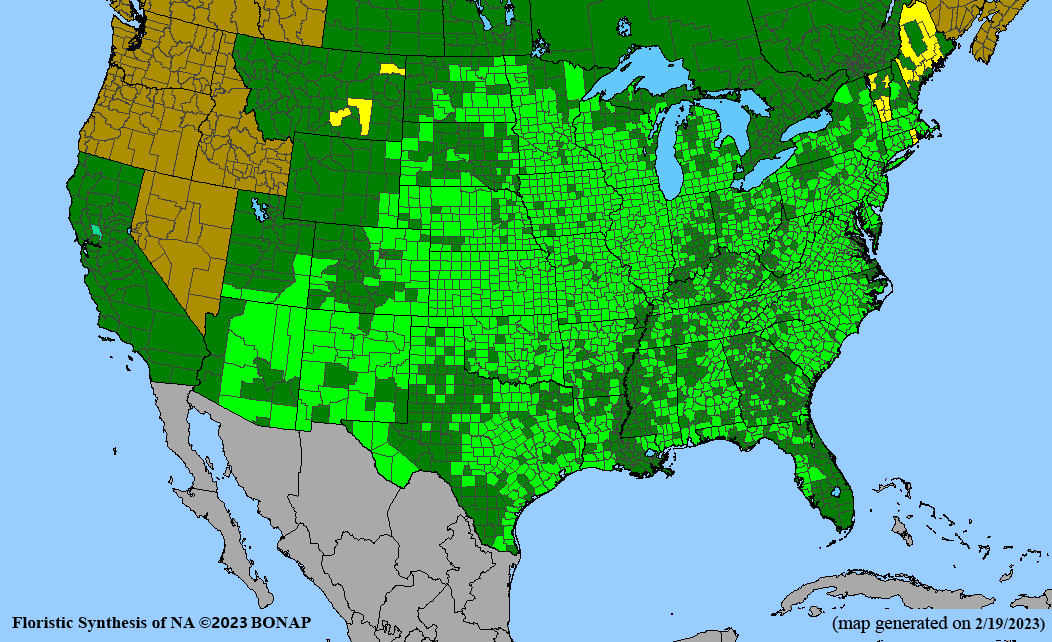
General Comments
Indiangrass is a dominant component of the tallgrass prairie ecosystem. This species generally establishes readily from seed, if good seed bed preparation and good weed control are achieved (i.e. following a glyphosate-resistant crop, for example). Two to three years are needed to develop a productive stand by direct seeding.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in Solid Stand PLS lbs/acre: 3.3 5.0 10 10-12 Seeding depth: 1/4-1/2 in
Seeding method: native seed drill
Seeding time: late spring to early summer.
Weed control: Prepare clean, very firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: No stratification necessary. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed in greenhouse two months before last frost free date at 1/4 in depth.
Transplanting: Transplant after all danger of frost.
- Stand management
Weeds: Mow stands high (6–12 in) first growing season to prevent weed canopy from shading seedlings. Established stands – Plateau (imazepic) for grass and broadleaf control, Atrazine for grass control. Always read and follow label instructions.
Pests: None noted.
Diseases: None noted.
- Seed production
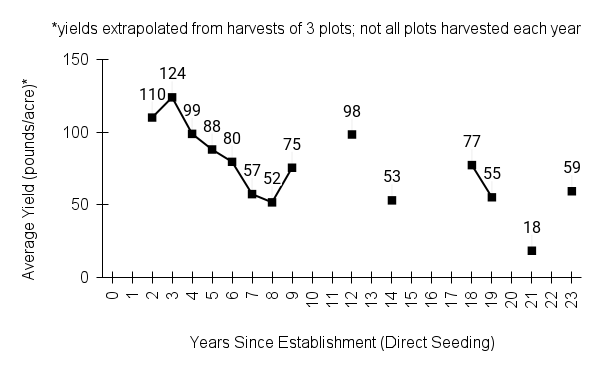 First harvest: Flowering and seed set at end of second growing season from direct seeding, three years for stand to fill out.
First harvest: Flowering and seed set at end of second growing season from direct seeding, three years for stand to fill out.Yield: 50-130 bulk pounds/acre (yields extrapolated from harvest records of three plots, not all of which were harvested every year)
Stand life: Peak harvests occur in the third year and after. If seed yields decline, stands can be chiselled to reinvigorate. Annual late spring fire helps control weeds and increase flowering and seed production. Fertilizer application may also improve seed yield. (Note: These recommendations are strictly for production fields, NOT REMNANT PRAIRIES). Productive stand life is 10-20 years.
Flowering date: Mid-August to mid-September.
Seed maturity/Harvest date: Late September to early October.
Seed retention: Shattering occurs soon after maturity. Very susceptible to seed shattering from wind. A single, windy afternoon when seed is mature and dry can take most of the crop. Monitor fields frequently. As seedheads near maturity, the awns and hairs fluff out. When this begins to happen, check for shattering and observe the stage of development of the grains.
Harvest date range at TPC (2003-2022): Sept 23 - Oct 21
Recommended harvest method: Seed stripper or combine at medium to hard dough stage.
- Seed cleaning and storage
Cleaning process: Air-dry material, remove awns with a debearder or brush machine, then air-screen. Protect eyes, airways, and skin from the irritating hairs released during harvest and cleaning processes.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project: Zone 1 (northern Iowa), Zone 2 (central Iowa), and Zone 3 (southern Iowa)
NRCS releases: Coastal Germplasm, Newberry Germplasm, Northern Missouri Germplasm, Southlow Michigan Germplasm, Suther Germplasm, Western Missouri Germplasm
Selected germplasm: Prairie View Indiana Germplasm (IN), Wynia Germplasm (AR, OK)
Informal: Cheyenne (OK)
Cultivated varieties (cultivars): Americus (AL, GA), Llano (NM), Lometa (TX), Osage (KS, OK), Rumsey (IL), Tomahawk (ND, SD); Horticultural varieties may also exist
- References
Chayka, K. (n.d.). Sorghastrum nutans (Indian grass). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/indian-grass
Hilty, J. (2019). Indian grass - Sorghastrum nutans. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/ind_grass.htm
Houseal, G. A. (2007). Grasses warm season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 74–75). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Sorghastrum nutans (L.) Nash. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SONU2
Species Guide Updated 12/11/2025
big bluestem
big bluestem sagem
Andropogon gerardii Vitman
Alternate Common Names: turkey foot, turkeyfoot
Scientific Synonyms: Andropogon chrysocomus Nash, Andropogon furcatus Muhl. ex Willd., Andropogon provincialis Lam., Andropogon gerardii Vitman var. chrysocomus (Nash) Fernald
Family: grass family (Poaceae)
Functional Group: warm season grasses
Description
- Life cycle and growth form
Perennial with short rhizomes and fibrous roots that forms large clumps, a bunchgrass.
Height: 2-8 ft
- Leaves and stem
Leaves flat with a prominent midrib, 1-2 ft long and 1/4 in wide, often with long, unkempt, white hairs near leaf base and on lower sheath, ligule is a short, fringed membrane; flowering culms (stems) are erect and hairless, solid, often reddish to bluish purple in color with a waxy bloom, usually with a few branches near the top.
- Flower, fruit and seedhead
Fruit/seedhead: Seed heads (panicles) consist of 2-6 spikelike racemes 1.5-4 in long at the tips of branches, containing both seed-bearing and sterile flowers; seed heads appear bristly when mature and shatter from the tops especially on dry, windy days.
Pollination: wind
- Seed
Seed characteristics
Seeds per ounce: 10,000 (IA NRCS)
Seeds per pound: 160,000 (IA NRCS)
1000 seed weight: 2.14 g (Seed Information Database)
Description: Fertile spikelet with awn, 1-2 cm long (1/2-3/4 in), attached stalk(s) are covered with hairs prior to debearding. Caryopsis smooth, brown, 3-5 mm long.
Typical seed test
PLS: 85%
Purity: 89%
Germination: 39%
Dormant: 56%
(averages obtained from 11 tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to wet-mesic soil; full sun; prairies, savannas, roadsides, fens, glades. Wetland Indicator Status is Facultative (FAC) for the Midwest. Moist, loamy, deep, well-drained soils are preferred for seed production.
Conservation status: Global- G5, secure; Wyoming- S3, vulnerable (NatureServe)
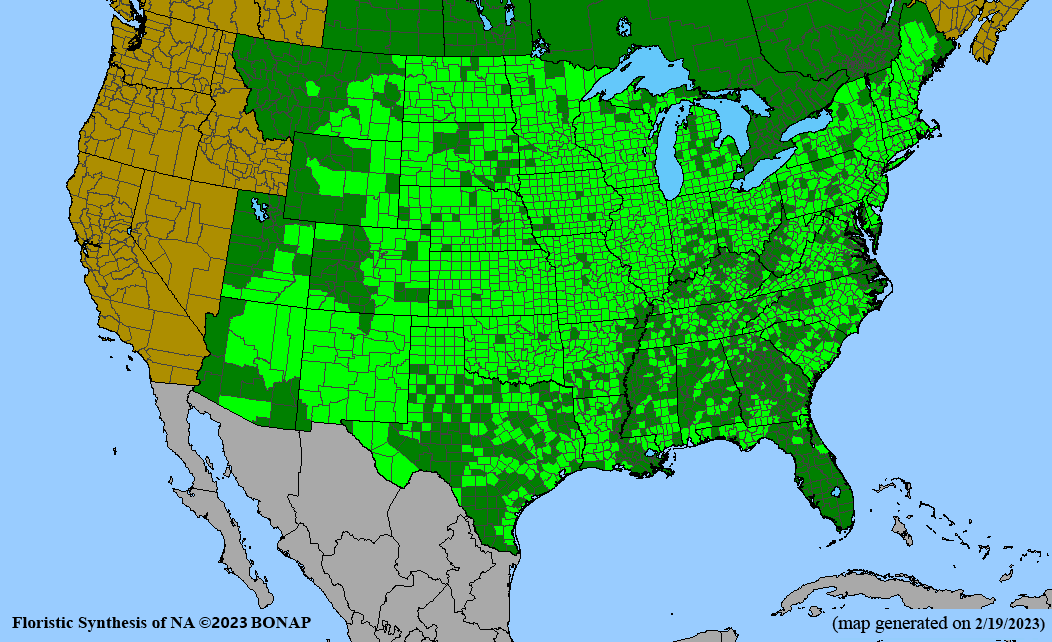
General Comments
Big bluestem is a dominant component of the tallgrass prairie ecosystem. This species establishes readily from direct seeding, particularly if seeded into crop ground where good weed control has been achieved (i.e. following a glyphosate-resistant crop, for example). It takes two to three years for the stand to develop, with good management and weed control.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in solid stand PLS lbs/acre: 3.6 4.8 9.7 10-12 Seeding depth:1/4-1/2 in
Seeding method: native seed drill
Seeding time: mid to late spring
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: No stratification necessary. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed in greenhouse 2 months before last frost free date at 1/4 in depth.
Transplanting: Transplant after all danger of frost.
- Stand management
Weeds: During establishment - mow the stand 6-12 in high during first growing season to prevent weed canopy from shading seedlings. Established stand – Atrazine, 2,4-D, Plateau (imazapic), Outlook (Dimethenamid-P).
Pests: Yellow midges may infest florets, reducing seed yields.
Diseases: Smut fungus affects florets.
- Seed production
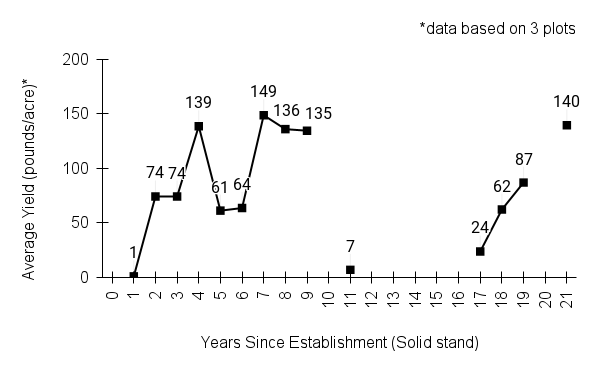
First harvest: Flowering and seed set end of second growing season from direct seeding, 3 years for stand to fill out.
Yield: 60-150 bulk pounds/acre (based on 3 solid stands with no supplemental nitrogen or irrigation; plots were not harvested every year)
Stand life: Peak harvests third year and after. If seed yields decline because stands are sod-bound, they can be chisel plowed to reinvigorate. Annual spring fire when green shoots are 2 in tall helps control weeds and increase flowering and seed production. (Note: This recommendation is strictly for production fields, not remnant prairies). Some producers use nitrogen application in spring to increase seed yield (60-100 pounds lb N/ac). Productive stand life 20 years or more.
Flowering date: early August - mid-September in northern Iowa
Seed maturity/Harvest date: October in northern Iowa
Seed retention: Shattering begins mid to late October.
Harvest date range at TPC (2003-2022): Sept 12 - Nov 2
Recommended harvest method: Combine at medium to hard dough stage, when some shattering is beginning to occur on the top of the main panicles.
- Seed cleaning and storage
Cleaning process: Air-dry material, scalp through 1/2 in mesh, remove awns with debearder or brush machine, then air-screen. Indent cylinder can help remove foxtail or similar weed seeds, if present.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Central (Zone 2), Northern (Zone 1), and Southern (Zone 3) Natural Selections/Iowa Ecotype Germplasm (IA), Northern Missouri Germplasm (MO), OH 370 Germplasm (OH), Southlow Michigan Germplasm (MI), Suther Germplasm (NC)
Selected germplasm: Bounty Germplasm (MN,SD), Hampton Germplasm (MO), OZ-70 Germplasm (AR, IL, MO, OK), Prairie View Indiana Germplasm (IN), Refuge Germplasm (AR, IL, MO, OK)
Cultivated variety (cultivar): Bison (ND), Bonilla (SD), Earl (TX), Kaw (KS), Niagara (NY), Rountree (IA), Sunnyview (SD)
- References
Chayka, K. (n.d.). Andropogon gerardii (big bluestem). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/big-bluestem
Hilty, J. (2019). Big bluestem - Andropogan gerardii. Illinois Wildflowers.https://www.illinoiswildflowers.info/grasses/plants/bigblue.htm
Houseal, G. A. (2007). Grasses warm season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 66–67). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA-NRCS. (n.d.). Conservation plant releases. Natural Resources Conservation Service. https://www.nrcs.usda.gov/plant-materials/cp/releases
USDA NRCS National Plant Data Team. (n.d.). Andropogon gerardii Vitman. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ANGE
Species Guide Updated 12/17/2024
composite dropseed
composite dropseed dickeye
Sporobolus compositus (Poir.) Merr.
Alternate Common Names: rough dropseed, tall dropseed, dropseed, flag grass
Scientific Synonyms: Sporobolus asper (P. Beauv.) Kunth
Family: grass family (Poaceae)
Functional Group: warm season grasses
Description
- Life cycle and growth form
Perennial from a fibrous root system, appearing as single stems or in loose clumps.
Height: 1.5-5 ft
- Leaves and stem
Leaf sheaths smooth except for hair tufts near junction with the blade (collar); leaf blades 3/16 in wide and up to 24 in long with tapering thread-like tip and rough upper surface; stems are stout and hairless, multiple stems from the base form a bunch.
- Flower, fruit and seedhead
Fruit/seed head: Narrow contracted spike-like panicle 4-8 in long, develops within sheath of uppermost leaf, only partially exposed at maturity.
Pollination: wind pollinated

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 30,000 (IA NRCS)
Seeds per pound 480,000 (IA NRCS)
1000 seed weight: 1.03 g (Seed Information Database)
Description: Spikelets are one-flowered, no awns present, 3.3-7 mm long (about 1/4 in). Grain is about 2 mm (1/16 in) in diameter, smooth, rounded, often dark brown.
Typical seed test
PLS: 94% (n = 11)
Purity: 100% (n = 11)
Germination: 60% (n = 9)
Dormant: 35% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to dry-mesic, on well-drained clay or silt loams; also on intermittently wet and dry sandy or rocky soils; full sun; prairies, savannas, roadsides, along railroads, fields, limestone glades; well-drained loamy soils preferred for seed production.
Conservation status: Global- G5, secure; Delaware and Montana- SH, possibly extirpated; West Virginia- S1, critically imperiled; Vermont and Wyoming- S2, imperiled (NatureServe)
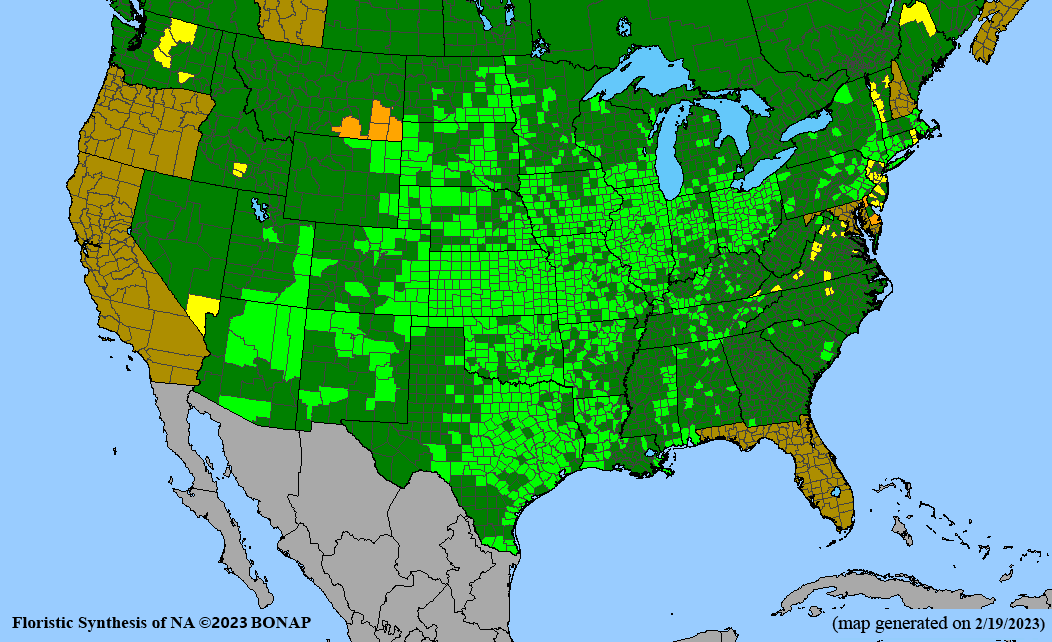
General Comments
Composite dropseed may be abundant on dry prairies as a bunchgrass or spreading by short rhizomes and is common on the shoulders of gravel roads in some areas. This species produces abundant seed, is very competitive when directly seeded into appropriate soils, and is relatively easy to harvest and clean. It has potential as an important nurse or cover crop for high diversity native plantings where quick establishment is needed and when seeding into warm soil.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in Solid Stand PLS lbs/acre: 1.2 1.8 3.6 3.6 Seeding depth: 1/4 in
Seeding method: native seed drill or broadcast for solid stand
Seeding time: late spring
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: No stratification necessary. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed in greenhouse two months before last frost free date at 1/4 in depth.
Transplanting: Transplant after all danger of frost.
- Stand management
Weeds: Mow stands high (6–12 in) first growing season to prevent weed canopy from shading seedlings. Herbicides include Outlook (dimethenamid-P) for grass control. Pendimax (pendimethalin) can be used to control broadleaf weeds in established stands. Always read label instructions.
Pests: None noted.
Diseases: None noted.
- Seed production
 First harvest: Flowering and seed set may occur at the end of the second growing season from direct seeding.
First harvest: Flowering and seed set may occur at the end of the second growing season from direct seeding.Yield: 120-250 bulk pounds/acre during peak production (per acre yields extrapolated based on production from 3 plots)
Stand life: Stand remains productive over several years, projected stand life 10-15 years.
Flowering date: Flowering begins in mid to late August in northern Iowa.
Seed maturity/Harvest date: Mid to late September in Northern Iowa.
Seed retention: Some shattering occurs soon after maturity.
Harvest date range at TPC (2003-2010): Sept 26 - Oct 27
Recommended harvest method: Composite dropseed has very tough stems and leafy material that may clog the sickle bar cutting head. Slow ground speed to compensate. Seed threshes fairly easily.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping thru 1/2 in and 1/4 in mesh if necessary to remove large particles. Glumes can be removed with a brush machine prior to air-screening if desired, or simply air-screen to clean.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project: Zone 1 (northern Iowa), Zone 2 (central Iowa), and Zone 3 (southern Iowa)
- References
Chayka, K. (n.d.). Sporobolus compositus (rough dropseed). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/rough-dropseed
Gleason, H. A., & Cronquist, A. (1991). Poaceae. In Manual of Vascular Plants of Northeastern United States and Adjacent Canada (2nd ed., p. 789). The New York Botanical Garden.
Hilty, J. (2019). Tall dropseed - Sporobolus compositus. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/tall_dropseed.htm
Houseal, G. A. (2007). Grasses warm season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 76–77). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Peterson, P. M., Hatch, S. L., & Weakley, A. S. (2021, May 11). Sporobolus compositus (Poir.) Merr. Flora of North America. http://floranorthamerica.org/Sporobolus_compositus
USDA NRCS National Plant Data Team. (n.d.). Sporobolus compositus (poir.) Merr. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SPCO16
Species Guide Updated 12/11/2025
little bluestem
little bluestem dickeye
Schizachyrium scoparium (Michx.) Nash
Alternate Common Names: prairie beardgrass, broom beardgrass
Scientific Synonym: Andropogon scoparius, Michx.
Family: grass family (Poaceae)
Functional Group: warm season grass
Description
- Life cycle and growth form
Perennial, short rhizomes, fibrous roots, grows in dense clumps.
Height: 1-3 ft

- Leaves and stem
Leaf blades narrow, up to 8 in long, flat or folded lengthwise, green to blue-green in color, usually hairless; sheaths strongly flattened and often hairless; ligule is a fringed (ciliate) membrane; nodes are hairless and purple; flowering stem is hairless and erect with many short branches, bluish to reddish-purple in color.
- Flower, fruit and seedhead
Fruit/seedhead: Single spikes, about 1 in long, arise from upper leaf axils, spikelets spread out as they mature, appearing as white, feathery appendages that arch; entire spikelets fall off at maturity and are weakly dispersed by the wind up to several feet from the parent plant.
Pollination: wind

- Seed
Seed characteristics
Seeds per ounce: 15,000 (IA NRCS)
1000 seed weight: 1.50 g (Seed Information Database)
Description: [shape, length, color, attached structures]
Typical seed test
PLS: 68% (n = 12)
Purity: 71% (n = 12)
Germination: 35% (n = 11)
Dormant: 59% (n = 11)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to dry-mesic soil; full sun; prairies, glades, dunes, roadsides, along railroads, woodland openings, scrubby barrens, abandoned fields; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; well-drained, moderately moist soils are preferred for seed production.
Conservation status: Global- G5, secure (NatureServe)
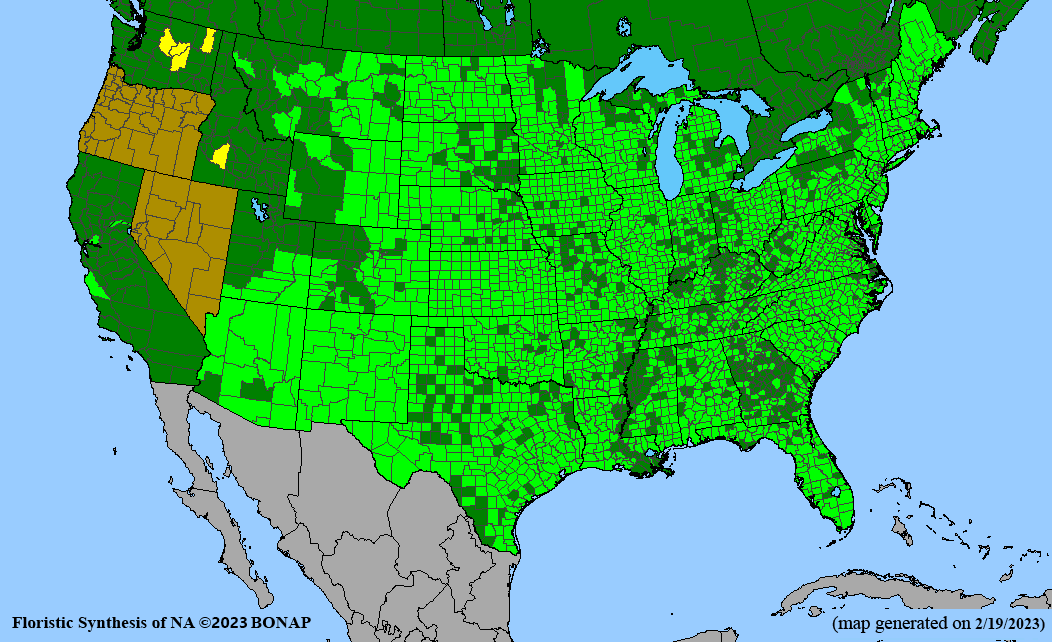
General Comments
Little bluestem is a dominant component on dry or well-drained soils within the tallgrass prairie region. Careful site selection, seedbed preparation, and weed control are critical to successful establishment from seed. No-till drilling with a native seed drill into cropland following a glyphosate-resistant crop, soybeans for example, is an excellent method. It takes two to three years for a stand to develop and reach peak yields.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in solid stand PLS lbs/acre: 2.4 3.2 6.4 8.0 Seeding depth: 1/4 in
Seeding method: native seed drill
Seeding time: late spring to early summer.
Weed control: Prepare clean, very firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: No stratification necessary. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed in greenhouse two months before last frost free date at 1/4 in depth.
Transplanting: Transplant after all danger of frost.
- Stand management
Weeds: Mow stands high (6–12 in) in the first growing season to prevent weed canopy from shading seedlings. Imazepic can be used to control grass and broadleaf weeds in established stands. Pre-emergent grass and broadleaf herbicides can be used for weed control. Always check chemical labels.
Pests: None noted.
Diseases: No significant issues noted in TPC production plots, however, a leaf spot fungus is known to infect little bluestem and related grass species.
- Seed production
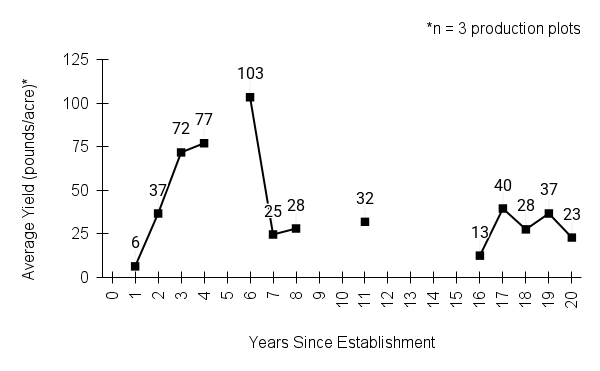 First harvest: Flowering and seed set end of second growing season from direct seeding, three years for stand to fill out.
First harvest: Flowering and seed set end of second growing season from direct seeding, three years for stand to fill out.Yield: 30-100 bulk pounds/acre
Stand life: Peak harvests third year and after. If seed yields decline, stands can be chiseled to reinvigorate. We do not apply fertilizer to TPC plots, but this may improve seed yield. Annual late spring fire helps control weeds and increase flowering and seed production. (Note: These recommendations are strictly for production fields, NOT REMNANT PRAIRIES). Productive stand life 10-20 years or more.
Flowering date: Late July to late August.
Seed maturity/Harvest date: Late September to October.
Seed retention: Shattering is moderate, beginning in late September. Much of the variation in seed yield at TPC appears to be due to harvest timing, particularly waiting too long.
Harvest date range at TPC (2003-2022): Sept 5 - Oct 29
Recommended harvest method: Stripper or combine at hard dough to maturity, when most spikes are fluffed out and shattering is just beginning to occur.
- Seed cleaning and storage
Cleaning process: Air-dry material, remove awns with a debearder or brush machine, then air-screen. Like other fluffy-seeded warm season grasses, this species may not flow particularly well through the air-screen cleaner.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Northern Iowa Germplasm (IA Zone 1), Central Iowa Germplasm (IA Zone 2), Southern Iowa Germplasm (IA Zone 3), Northern Missouri Germplasm, Southern Missouri Germplasm, Southlow Michigan Germplasm, Suther Germplasm
Selected germplasm: Badlands Ecotype (ND, SD), Coastal Plains Germplasm (TX, LA), Itasca Germplasm (ND), OK Select Germplasm (OK), Ozark Germplasm (MO, IL), Prairie View Indiana Germplasm (IN), STN-176 Germplasm (TX), STN-461 Germplasm (TX)
Cultivated varieties (cultivars): Ahring (Great Plains), Aldous (KS), Cimarron (OK, KS), Pastura (NM), Sims (Great Plains)
- References
Chayka, K. (n.d.). Schizachyrium scoparium (little bluestem). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/little-bluestem
Hilty, J. (2019). Little bluestem - Schizachyrium scoparium. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/little_bluestem.htm
Houseal, G. A. (2007). Grasses warm season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 72–73). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Schizachyrium scoparium (Michx.) Nash. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SCSC
USDA NRCS. Prairie View Indiana Germplasm Little Bluestem [Infographic] Retrieved February 20, 2025 from https://efotg.sc.egov.usda.gov/references/public/IN/Revised_Prairie_View_Little_bluestem.pdf
Wipff, J. K. (2021, May 11). Schizachyrium scoparium (Michx.) Nash. Flora of North America. http://floranorthamerica.org/Schizachyrium_scoparium
Species Guide Updated 11/17/2025
prairie cordgrass
prairie cordgrass dickeye
Spartina pectinata Bosc ex Link
Alternate Common Names: slough grass, marshgrass, ripgut, fresh water cordgrass
Scientific Synonyms: Sporobolus michauxianus (Hitchc.) P.M. Peterson & Saarela, Spartina michauxiana Hitchc., Spartina pectinata Bosc ex Link var. suttiei (Farw.) Fernald
Family: grass family (Poaceae)
Functional Group: warm season grass
Description
- Life cycle and growth form
Perennial warm-season grass from stout, scaly, sharply pointed rhizomes, forming large, dense, clonal colonies.
Height: 3-8 ft

- Leaves and stem
Leaf blades 6-15 mm wide (up to just over 1/2 in) and 20-120 cm long (8 - 48 in) with coarsely serrate margins that can cause minor lacerations on exposed skin, sheaths smooth with visible longitudinal veins; flowering stems (culms) are erect and hairless.
- Flower, fruit and seedhead
Fruit/seed head: Seedhead is a raceme 4-15 cm long (2-6 in) with 10-30 densely packed, one-sided spikes each containing 10-25 flattened spikelets. The entire spikelet sheds at maturity, leaving a naked stalk.
Pollination: wind

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 6,600 (IA NRCS)
1000 seed weight: 1.85 g (Seed Information Database)
Description: One-flowered spikelet with short awn (less than 1/4 in). Seeds are very flat. Caryopsis is about 5 mm long.
Typical seed test
PLS: 71%
Purity: 75%
Germination: 20%
Dormant: 50%
(averages obtained from 4 tests of purchased seed lots)
- Habitat and range
Habitat: Wet-mesic to wet soil; full sun; swales, roadside ditches, marshy areas, drainage areas, and wetlands; it will grow on seasonally dry sites, but won’t tolerate prolonged flooding; Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest; irrigation is necessary for seed production plots.
Conservation status: Global- G5, secure; Delaware, District of Columbia,Georgia, and North Carolina- S1, critically imperiled; Louisiana, Oregon, Virginia, and Washington- S2, imperiled; Kentucky- S2/S3, imperiled to vulnerable (NatureServe)
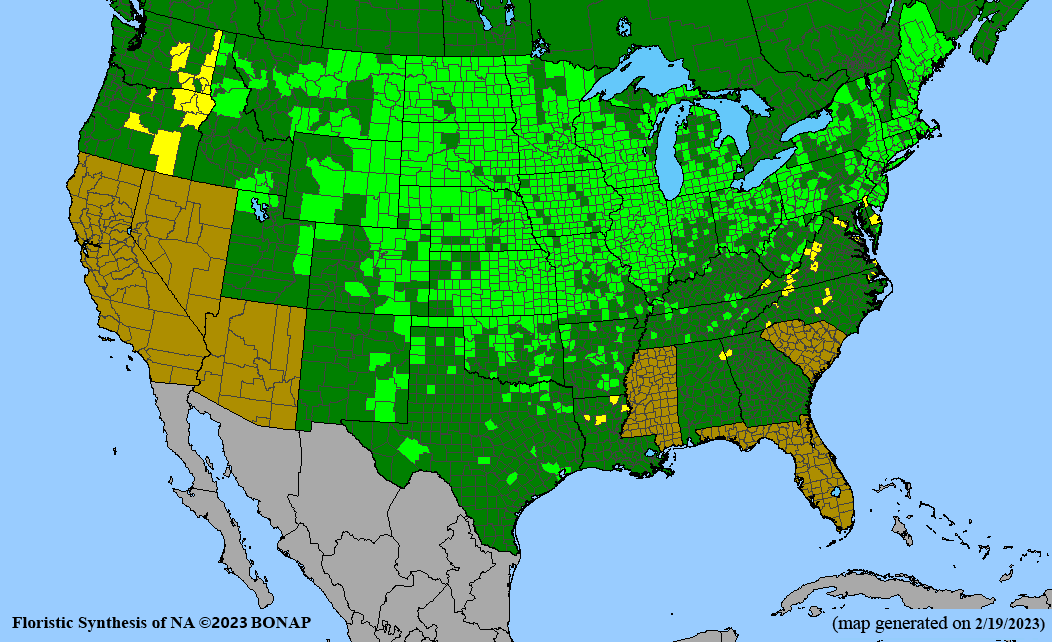
General Comments
Cordgrass has a reputation for poor seed production. Its primary mode of growth is vegetative spread by rhizomes. Cordgrass often forms large, dense colonies with few flowering stalks, and these mostly situated on the outer, leading edges of the colony. Insect predation of the seed heads further limits seed production from native stands. Yet cordgrass does grow readily from viable seed in plantings. Direct seeding for a seed increase stand, however, is not recommended.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: Moist stratify seed for up to 4 weeks, or soak in water for 24 hours and freeze overnight to improve germination.
Sowing: Sow seed in greenhouse two months before last frost free date at 1/2 in depth.
Transplanting: Transplant after all danger of frost. Greenhouse grown plugs can be transplanted into wide row spacing, 6-8 ft between rows, with plants 2-3 ft apart within the rows. This gives the newly established plants adequate space for rhizome spread and promotes more flowering and seed set after establishment.
- Stand management
Weeds: Pre-emergent herbicides can be used after transplanting seedling plugs or pieces of rhizome. It’s critical to water-in transplants to seal soil around roots to prevent herbicide from coming into contact with and possibly damaging roots. Read and follow label instructions.
Pests: There are several species of host-specific insects that use Spartina including a moth larva that feeds within developing seed heads, and these can be destructive in seed production settings. These can reportedly be controlled with insecticide application during flowering. There is also reportedly less predation when grown in northern regions (e.g. North Dakota) (USDA-NRCS Plant Guide).
Diseases: None noted.
- Seed production
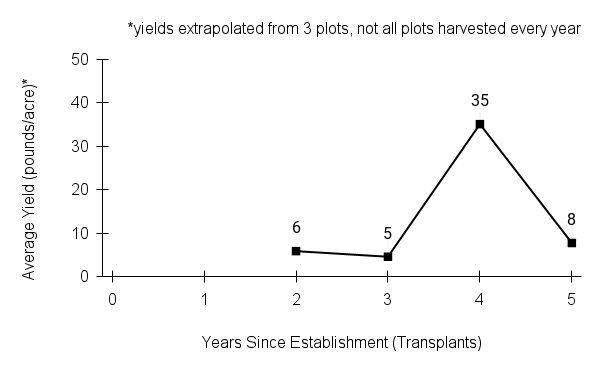 First harvest: Some flowering and seed set occurs in the second growing season from greenhouse grown transplants. Seed production may occur the first year from transplanted rhizomes.
First harvest: Some flowering and seed set occurs in the second growing season from greenhouse grown transplants. Seed production may occur the first year from transplanted rhizomes.Yield: 10-30 bulk pounds/acre at TPC. Other growers report yields of 30 to 75 pounds/acre (USDA-NRCS Plant Guide).
Stand life: Irrigation is critical to successful establishment and good seed production of this species over the first few years of stand life. Expect 2-3 years for the stand to be established. Stand may become root-bound by the fifth year, and seed production declines, at which time a new stand should be established from rhizomes.
Flowering date: Mid-July to early September.
Seed maturity/Harvest date: Late September to early October.
Seed retention: Shattering occurs from mid to late October.
Harvest date range at TPC (2003-2007): Sept 28 - Nov 3
Recommended harvest method: Combine at maturity and before shattering. (No combine settings available in Appendix).
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping thru 1/2 in and 1/4 in mesh to remove large particles. Remove awns with debearder. Seeds are long and flat, and easily damaged by a brush machine. Air-screen to clean. Refer to Appendix C for settings.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zone “Iowa”
Selected germplasm: Atkins Germplasm (NE), Kingston Germplasm (MA, ME, NH), Red RIver Natural Germplasm (MN, ND, SD), Southampton Germplasm (NY)
- References
Chayka, K. (n.d.). Spartina pectinata (prairie cordgrass). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/prairie-cordgrass
Hilty, J. (2019). Prairie cordgrass - Spartina pectinata. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/pr_cordgrass.htm
Houseal, G. A. (2007). Grasses warm season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 80–81). Tallgrass Prairie Center - University of Northern Iowa.
Jensen, N.K. (2017, September 13) Prairie Cordgrass Spartina pectinata Bosc ex Link. USDA NRCS Plant Guide. https://www.nrcs.usda.gov/plantmaterials/nypmcpg11942.pdf
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Spartina pectinata Bosc ex Link. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SPPE
Species Guide Updated 12/15/2025
prairie dropseed
prairie dropseed dickeye
Sporobolus heterolepis (Gray) Gray
Alternate Common Name: northern dropseed
Family: grass family (Poaceae)
Functional Group: warm season grasses
Description
- Life cycle and growth form
Perennial warm-season grass forming long-lived dense bunches of many stems that become hummocky over time.
Height: 1-3 ft

- Leaves and stem
Leaves with hairless blades about 1/16 in wide and up to 2 ft long, tapered to a thread-like tip; leaf sheath smooth except for a tuft of hairs at the collar; stems are slender and hairless.
- Flower, fruit and seedhead
Fruit/seed head: Seedhead is a diffuse, openly branched panicle. Glands at the base of branches in the panicle give off a somewhat rancid buttery odor when in flower and setting seed.
Pollination: Wind-pollinated, though small flower flies visit the flowers to feed on pollen.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 16,000 (IA NRCS)
1000 seed weight: 1.90 g (Seed Information Database)
Description: Spikelets are one-flowered, no awns present, about 4 mm long (5/32 in), grain round, firm about 2.5 mm (3/32 in) long.
Typical seed test
PLS: 86% (n = 10)
Purity: 96% (n = 10)
Germination: 20% (n = 6)
Dormant: 55% (n = 7)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to moist soil; full sun; high quality remnant prairies, limestone glades, savannas; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest.
Conservation status: Global- G5, secure; Massachusetts- SH, possibly extirpated; Wyoming, Connecticut, Georgia, Kentucky, Maryland, North Carolina, Ohio, Pennsylvania, Tennessee, and Virginia- S1, critically imperiled; New York and Oklahoma- S2, imperiled; Illinois- S2/S3, Colorado, Kansas, and Michigan- S3, vulnerable (NatureServe)

General Comments
This species is seldom abundant in prairies, occurring in groupings of scattered clumps. The long, slender leaves, bunching habit, and airy seedheads create a fountain-like effect, making this species desirable for horticultural landscape plantings. Seedlings develop slowly, so this species is best propagated in the greenhouse and transplanted in rows convenient for tillage equipment in a well-prepared, weed-free, and firmly packed increase field. Plants are very long-lived, forming large clumps after 2-3 growing seasons. Spring burning stimulates prolific flowering and seed production, but bunches can also be killed or damaged by burning if soil conditions are excessively dry. Timing of seed harvest is critical, since seed drops soon after maturity.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species due to slow seedling development
Greenhouse
Seed pre-treatment: Moist stratify seed at 35-40° F for 4 weeks.
Sowing: Sow seed in greenhouse 2 months before last frost-free date at 1/4 in depth. Warm temperatures in the greenhouse appear to improve germination.
Transplanting: Transplant (after all danger of frost) into rows convenient for tillage equipment or into weed barrier or plastic mulch at 8-12 in spacing.
- Stand management
Weeds: Transplant into well-prepared, weed-free increase field. Pre-emergent herbicides may be used after transplanting. Be sure to water in transplants to help seal soil around roots so pre-emergent won’t chemically damage root systems. Cultivate, hoe, and hand rogue around young plants later in the season, if necessary. Weed barrier or plastic mulch, if used, suppresses weeds in the first year or two, and buildup of thatch between burnings suppresses some weeds. Remove plastic to allow burning in subsequent years. Some native seed producers use a companion planting such as buffalo grass to suppress weeds between prairie dropseed rows.
Pests: Voles like to nest in prairie dropseed thatch and will cut and eat (or store) the base of flowering stems before seed matures, potentially decimating yields.
Diseases: None noted.
- Seed production
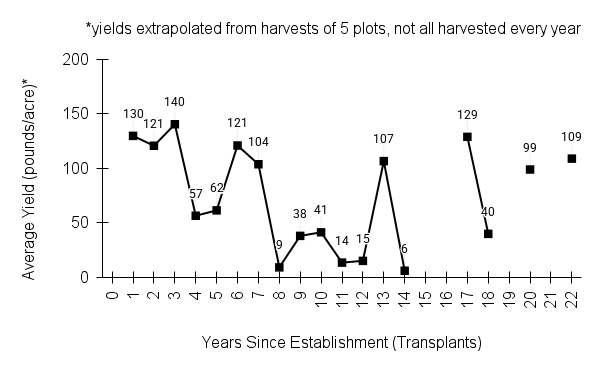 First harvest: Flowering and seed set occurs at the end of the second growing season from greenhouse grown transplants.
First harvest: Flowering and seed set occurs at the end of the second growing season from greenhouse grown transplants.Yield: Typically around 50-150 bulk pounds/acre with much year to year variation (per acre yields extrapolated based on harvests from 5 plots). Spring burning may stimulate flowering and seed set, though bunches can be killed by fire if soil is very dry.
Stand life: Stands remain productive for 10-20 years, although yields fluctuate greatly.
Flowering date: mid-August - early September in northern Iowa
Seed maturity/Harvest date: late September - early October in northern Iowa
Seed retention: Shattering occurs soon after maturity. Check plots frequently as seed matures. A useful method to determine maturity is to crush seeds and observe for a crumbly, starchy center (no longer milky or creamy). The taste also changes from slightly sweet to starchy as seed matures.
Harvest date range at TPC (2003-2023): Sept 22 - Oct 18 (harvests after this date produced very little seed)
Recommended harvest method: combine
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping through 1/2 in and 1/4 in mesh to remove large particles, if necessary. Air-screen to clean.
Seed storage: Seed reportedly has a high oil content that can shorten viability in storage. Some producers recommend storing seed at freezing temperatures for extended storage after proper drying and cleaning. Store seed in moisture-proof containers before freezing. Seed should not be left at room temperatures for more than a few weeks after harvest. Refrigerated conditions (33-50° F, 30-50% RH) are adequate for at least a year after harvest.
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern Iowa), Zone 2 (central Iowa), and Zone 3 (southern Iowa).
Cultivated varieties (cultivars): Horticultural varieties selected for particular traits include Tara, Morning Mist, and Wisconsin Strain.
- References
Chayka, K. (n.d.). Spororbolus heterolepis (prairie dropseed). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/prairie-dropseed
Hilty, J. (2019). Prairie dropseed - Sporobolus heterolepis. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/pr_dropseed.htm
Houseal, G. A. (2007). Grasses warm season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 78–79). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Sporobolus heterolepis (A. Gray) A. Gray. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SPHE
Species Guide Updated 12/11/2025
sideoats grama
sideoats grama dickeye
Bouteloua curtipendula (Michx.) Torr.
Alternate Common Names: sideoats, side oats grama, side-oats grama
Scientific Synonym: Atheropogon curtipendula
Family: grass family (Poaceae)
Functional Group: warm season grasses
Description
- Life cycle and growth form
Perennial, warm-season bunchgrass, spreading slowly by short rhizomes to form loose colonies.
Height: 1-3 ft

- Leaves and stem
Leaves mostly basal with leaf blades 6-8 in long and tapered to a sharp point; stiff hairs with glandular bases evenly spaced along the leaf margins, sticking out at a right-angle from the main axis of the blade; lower leaves curl and turn a light, tawny color when dry; ligule a very short fringe of hair; nodes hairless and green to purple; flowering stem is smooth and erect.
- Flower, fruit and seedhead
Fruit/seedhead: Seed head 4-12 in long consisting of many short oat-like spikes (0.5-1.25 in long), each with 3-7 spikelets all turned to one side of the main stem, giving rise to the common name “sideoats;” entire spike falls when mature, leaving a naked stalk with visible nodes.
Pollination: Wind

- Seed
Seed characteristics
Seeds per ounce: 6,000 (IA NRCS)
Seeds per pound: 96,000 (IA NRCS)
1000 seed weight: 1.30 g (Seed Information Database)
Description: Seed unit is a whole spike, fragment of spike, or floret. Although a seed unit may contain more than one germinable seed, it is counted as a single live seed in the calculation of pure live seed. Caryopsis (grain) 3-4 mm long, smooth brown.
Typical seed test
PLS: 89% (n = 12)
Purity: 96% (n = 12)
Germination: 59% (n = 11)
Dormant: 34% (n = 11)
(average of n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to mesic, fine textured, calcium-rich soil; full sun; prairies, bluffs, along railroads, woodland openings. Well-drained soils are preferred for seed production.
Conservation status: Global- G5, secure; District of Columbia- SH, possibly extirpated; Connecticut, Louisiana, Florida, and Michigan- S1, critically imperiled; Georgia, Maryland, Nevada, New York, and Pennsylvania- S2, imperiled; Indiana and West Virginia- S3, vulnerable (NatureServe)
General Comments
Sideoats grama is an important component of tall and mixed-grass prairies, occurring on well-drained, dry, rocky, alkaline soils. This species establishes readily from direct seeding, particularly if seeded into crop ground where good weed control has been achieved (following a glyphosate-resistant crop, for example). The foliage provides forage for mammalian herbivores as well as specialist and generalist insects, and grassland birds feed on the seeds.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in solid stand PLS lbs/acre: 3 4 8 9 Seeding depth: 1/4-1/2 in
Seeding method: native seed drill
Seeding time: Late spring when soil temperature reaches 55° F.
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: No stratification necessary. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed in greenhouse two months before last frost free date at 1/4 in depth.
Transplanting: Transplant into prepared beds at 12 in spacing after all danger of frost.
- Stand management
Weeds: Mow stands high (6-12 in) in first growing season to prevent weed canopy from shading seedlings. Do not use atrazine the year of establishment. On established stands, Plateau (imazapic); Outlook (dimethenamid-P), and 2,4-D have been used. Hand roguing removes weeds that could contaminate seed, cultivation or mowing can be used between rows, and burning in late spring helps control cool season weeds and may prevent buildup of disease inoculum.
Pests: Gall midge larvae have been observed within spikelets.
Diseases: Stem and leaf rust and other fungi are known to occur.
- Seed production
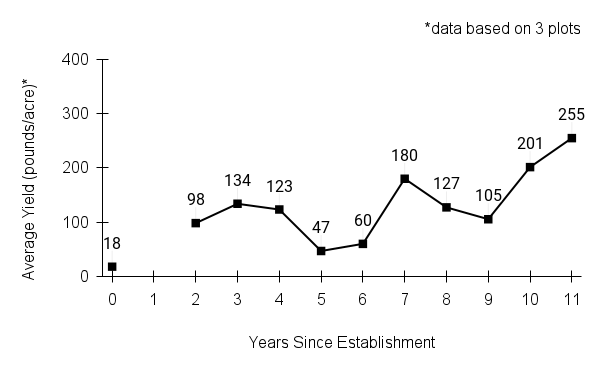
First harvest: Flowering and seed set end of second growing season from greenhouse grown transplants.
Yield: 20-255 bulk pounds/acre (per acre yields extrapolated based on production from 3 plots)
Stand life: Peak harvests third year and after. Annual late spring fire when shoots are 1 in tall helps control weeds and increase flowering and seed production. (Note: This recommendation is strictly for production fields, not remnant prairies.) Stand should persist 10 years or more if properly matched to soils and well managed.
Flowering date: mid-June - early July in northern Iowa
Seed maturity/Harvest date: September in northern Iowa
Seed retention: Holds seed fairly well, shattering occurs in October. Monitor fields fairly often as they mature and consider harvest when about 10% of stems have lost some spikelets from the top.
Harvest date range at TPC (2003-2023): Sept 1 - Oct 30
Recommended harvest method: Combine at hard dough stage.
- Seed cleaning and storage
Cleaning process: Combine-harvested sideoats grama can be air screened initially to sort spikelets from plant fragments. Larger intact spikes can be run quickly through a debearder or hammer mill to break up spikes, and re-air screen. Indent to remove foxtail or other short-seeded weeds.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Central Iowa Germplasm (IA Zone 2), Northern Iowa Germplasm (IA Zone 1), Southern Iowa Germplasm (IA Zone 3), Northern Missouri Germplasm
Selected Germplasm: South Texas Germplasm (TX)
Cultivated variety (cultivar): Midwest adapted cultivars include El Reno (KS), Haskell (TX), Niner (NM), Pierre (ND), Premier (TX), Vaughn (NM).
Informal Variety: Killdeer (ND)
- References
Chayka, K. (n.d.). Bouteloua curtipendula (side-oats grama). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/side-oats-grama
Gleason, H. A., & Cronquist, A. (1991). Poaceae. In Manual of Vascular Plants of Northeastern United States and Adjacent Canada (2nd ed., pp. 795-796). The New York Botanical Garden.
Hilty, J. (2019). Side oats grama - Bouteloua curtipendula. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/so_grama.htm
Houseal, G. A. (2007). Grasses warm season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 68–69). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Bouteloua curtipendula (Michx.) Torr. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=BOCU
Species Guide Updated 12/13/2024
switchgrass
switchgrass dickeye
Panicum virgatum, (L.)
Alternate Common Names: thatchgrass, Wobsqua grass, blackbent, tall panic grass, old switch panicgrass
Scientific Synonyms: Panicum virgatum var. spissum, Panicum virgatum var. cubense, Panicum bavardii
Family: grass family (Poaceae)
Functional Group: warm season grass
Description
- Life cycle and growth form
Warm season perennial, rhizomatous, forms clonal patches with many stems that expand over time.
Height: 3-6 ft
- Leaves and stem

Leaf blades 5/16 in wide and 6-22 in long, often hairy on the upper surface, especially near the ligule, ligule is fringe of dense hairs about 1/8 in tall; stem erect and hairless.
- Flower, fruit and seedhead
Fruit/seed head: Seedhead is an openly branched, airy panicle 8-16 in long with green to purple spikelets near the ends of the branches.
Pollination: wind
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 14,000 (IA NRCS)
Seeds per pound: 259,000 (IA NRCS)
1000 seed weight: 1.30 g (Seed Information Database)
Description: Spikelet is two-flowered with the fertile floret uppermost, smooth, awnless. Grain is shiny, smooth, 3-4 mm (about 1/8 in) long.
Typical seed test
PLS: 91% (n = 9)
Purity: 97% (n = 9)
Germination: 49% (n = 7)
Dormancy: 44% (n = 7)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to wet-mesic soil; full sun; prairies, savannas, streambanks, shorelines, dunes, woodland openings, roadsides, along railroads, ditches; may become abundant in disturbed prairies, much less common in high quality prairies. Wetland Indicator Status is Facultative (FAC) for the Midwest. Fertile, well-drained soils are preferred for seed production.
Conservation status: Global- G5, secure; Nevada- S2, imperiled; Vermont and Wyoming- S3, vulnerable (NatureServe)
General Comments
A number of cultivars of switchgrass have been developed for forage and seed production, winter hardiness, and grazing tolerance by the USDA-NRCS Plant Materials program. These cultivars have been planted widely as monocultures and in early prairie reconstructions. Because seed has been commercially available at affordable prices for decades, it was usually seeded heavily and tended to dominate stands. For these reasons it has been considered aggressive. Switchgrass can form dense colonies on lowland prairies, but is usually uncommon on high-quality remnant upland prairies and tends to occur in isolated patches near disturbance activities such as gopher mounds (Weaver 1954). Switchgrass establishes readily from seed, and is relatively easy to harvest and clean.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in Solid Stand PLS lbs/acre: 2.6 3.5 6.0 6.0 Seeding depth: 1/4 in
Seeding method: Native seed drill or broadcast seed and cultipack for solid stand.
Seeding time: Spring
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: Moist stratify seed for 4 weeks to improve germination.
Sowing: Sow seed in greenhouse two months before the last frost free date at 1/4 in depth.
Transplanting: Transplant after all danger of frost into rows convenient for tillage equipment.
- Stand management
Weeds: Mow stands high (6-12 in) first growing season to prevent weed canopy from shading seedlings. Broadleaf herbicides can be used to control broadleaf weeds in established stands. Switchgrass is atrazine resistant, and can be applied at the label rate at planting time. Read and follow label instructions.
Pests: None noted.
Diseases: Seed smut, if left unchecked, can seriously decrease seed yields on switchgrass. The smut is caused by a fungus, Tilletia maclaganii. Glumes may exhibit an uncharacteristic purple coloration, and seeds are replaced by fungal spores that are red-orange when immature turning dark brown at maturity. Fields may need to be destroyed or relocated if diseased (NRCS 2003).
- Seed production
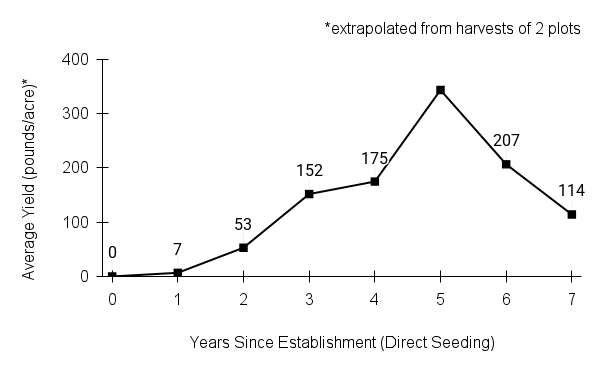 First harvest: Flowering and seed set end of first growing season from greenhouse grown transplants, second growing season from direct seeding.
First harvest: Flowering and seed set end of first growing season from greenhouse grown transplants, second growing season from direct seeding.Yield: 150-350 bulk pounds/acre (per acre yields extrapolated based on harvests of 2 plots)
Stand life: Stands should persist 10-15 years or more. Good seed production second year and after.
Flowering date: late July - early September in northern Iowa
Seed maturity/Harvest date: September in northern Iowa
Seed retention: Shattering begins in late September to early October
Harvest date range at TPC (2003-2010): Sept 16 - Nov 2
Recommended harvest method: Combine at hard dough stage before significant shattering has occurred.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping thru 1/2 in and 1/4 in mesh to remove large particles. Brush to remove all floral parts from the grain, air-screen to clean.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Projects Zones 1 (northern Iowa), 2 (central Iowa), and 3 (southern Iowa)
Cultivated varieties (cultivars): Mid-west adapted include Blackwell (KS), Cave-In-Rock (IL), Dacotah, Forestburg (ND), Nebraska 28 (NE), Shawnee (MO)
- References
Chayka, K. (n.d.). Panicum virgatum (switchgrass). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/switchgrass
Freckmann, R. W. & Lelong, M. G. (2021, May 11). Panicum virgatum L. Flora of North America. http://floranorthamerica.org/Panicum_virgatum
Hilty, J. (2019). Switch grass - Panicum virgatum. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/switchgrass.htm
Houseal, G. A. (2007). Grasses warm season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 70–71). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Panicum virgatum L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=PAVI2
Species Guide Updated 12/5/2025
Cool Season Grasses
Cool Season Grasses sagemThe Species Production Guides for cool season grasses provide specific information about growing each of these species for seed production.
A printable file (pdf) is also provided on each species page for those needing a print version.
This section is a work in progress. We will continue to add new species guides as they are completed.
Festuca paradoxa / clustered fescue
Glyceria striata / fowl mannagrass
Hesperostipa spartea / porcupinegrass
Canada wildrye
Canada wildrye dickeye
Elymus canadensis L.
Alternate Common Name(s): nodding wildrye, western wildrye, great plains wildrye
Scientific Synonym(s): Elymus brachystachys Scribn. & C.R. Ball, Elymus canadensis L. var. brachystachys (Scribn. & C.R. Ball) Farw., Elymus canadensis L. var. hirsutus (Farw.) Dorn, Elymus canadensis L. var. robustus (Scribn. & J.G. Sm.) Mack. & Bush, Elymus crescendus L.C. Wheeler, Elymus philadelphicus L., Elymus philadelphicus L. var. hirsutus Farw., Elymus robustus Scribn. & J.G. Sm.
Family: grass family (Poaceae)
Functional Group: cool season grasses
Printable PDF Elymus canadensis
PDF will be added soon
Description
- Life cycle and growth form
Perennial, cool-season bunchgrass, weak to no rhizomes.
Height: 1-5 ft
- Leaves and stem
Leaf blades up to 16 in long, 1/4-3/4 in wide, ligule a short (up to 1 mm) membrane, sheaths usually smooth with two small, purplish to brown lobes (auricles) clasping stem where the sheath meets the blade, nodes hairless and hidden under the sheaths; stems smooth, erect, unbranched.
- Flower, fruit and seedhead
Fruit/seedhead: Seedhead a thick, bristly spike, 3-10 inches long, usually nodding, light tan when mature, each spikelet with a pair of awned glumes (up to 1.5 in including the awn) and 3-5 florets, lemmas (chaffy parts around the grain) also awned (up to 2.5 in including the awn), awns twist and curve outward when dry, florets drop when mature, leaving glumes on the stalk.
Pollination: wind
- Seed
Seed characteristics
Seeds per ounce: 5,200 (IA NRCS)
Seeds per pound: 83,200 (IA NRCS)
1000 seed weight: 4.50 g (Seed Information Database)
Description: The long, barbed awns make this species difficult to clean. Curved awns on lemmas are up to 5 cm (2 in). Glumes taper to awns 1-3 cm (1/2-1.25 in) long. Caryopsis dark brown at maturity, 5-8 mm long.
Typical seed test
PLS: 88%
Purity: 96%
Germination: 66%
Dormant: 19%
(averages obtained from 12 tests of purchased seed lots)
- Habitat and range
Habitat: Broadly adapted to a range of conditions: dry to moist soil; partial to full sun; prairies, savannas, woodland edges, bluffs, dunes, riverbanks, upland and lowland, open areas, disturbed areas. Wetland Indicator Status is Facultative Upland (FACU) for the Midwest. Well-drained, loamy soils are preferred for production.
Conservation status: Global- G5, secure; Maine- SH, possibly extirpated; Nevada and Virginia- S1, critically imperiled (NatureServe)
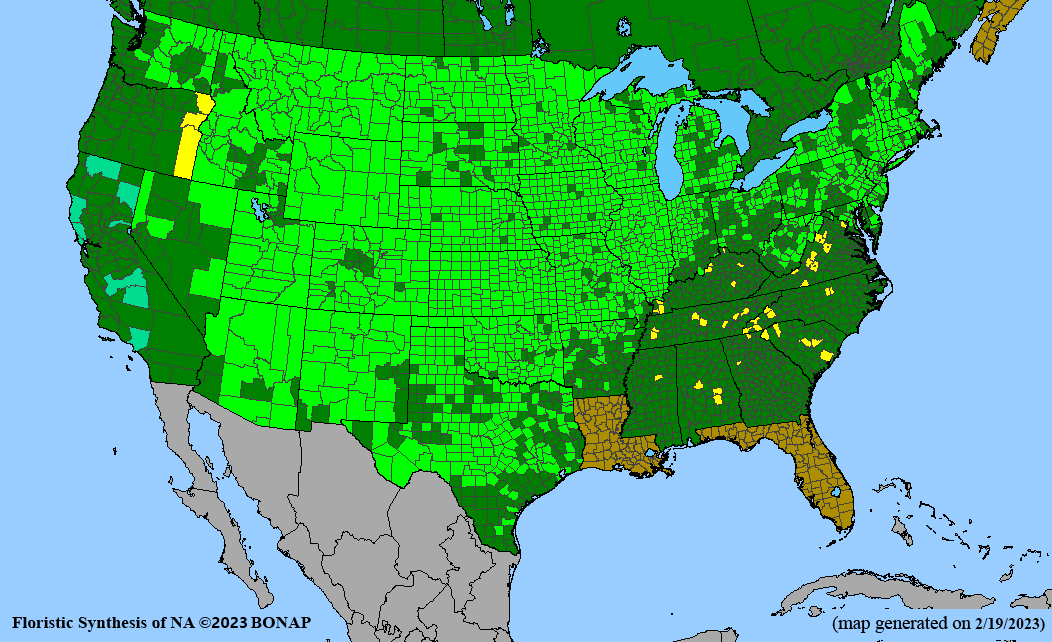
General Comments
Canada wildrye is a relatively short-lived perennial bunchgrass which establishes readily from seed in mixed plantings. These two traits make it ideally suited as a nurse crop for prairie restorations. It can also be direct-seeded as a seed production field into a well-prepared, weed-free seed bed (e.g., following a glyphosate-resistant crop).
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in solid stand PLS lbs/acre: 7 10.5 21 21 Seeding depth: 1/4-1/2 in
Seeding method: native seed drill
Seeding time: Fall, or early spring preferred.
Weed control: Prepare clean, firm, weed free seedbed prior to seeding.
Greenhouse
Seed pre-treatment: No stratification necessary. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed in greenhouse two months before last frost free date at 1/4 in depth.
Transplanting: Transplant at 12 in spacing in prepared beds after all danger of frost.
- Stand management
Weeds: Mow stands high (6–12 in) first growing season to prevent weed canopy from shading seedlings. Broadleaf herbicides can be used to control broadleaf weeds in established stands.
Pests: None noted.
Diseases: Susceptible to leaf and stem rust, also ergot.
Hybridization risk: This species is known to hybridize with related species Elymus hystrix, E. villosus, E. virginicus
- Seed production
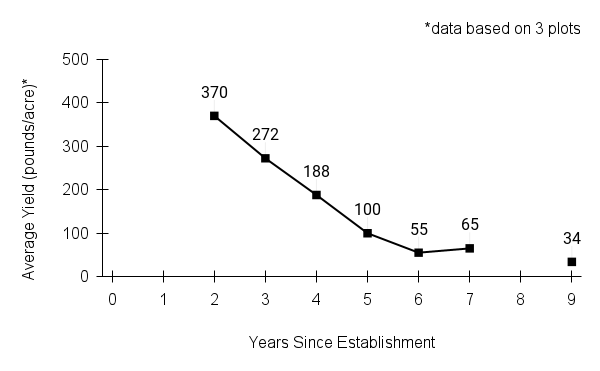
First harvest: Flowering and seed set will occur at the end of the first growing season from previous fall seeding or early spring seeding, or late spring transplants.
Yield: 35-370 bulk pounds/acre (per acre yield extrapolated from 3 plots)
Stand life: 4-6 years. Seed production declines significantly in the fifth year and after. Annual fall burning will prolong stand life and seed yield.
Flowering date: mid-July to mid-August in northern Iowa
Seed maturity/Harvest date: September in northern Iowa
Seed retention: shattering occurs early to mid-October
Harvest date range at TPC (2003-2010): Sept 7 - Nov 4
Recommended harvest method: Combine at maturity (hard dough stage). Long, barbed awns make harvesting a challenge, causing seed to ball up and not flow. Additional de-awning bars or other modifications to the combine may be required for successful harvest of this species.
- Seed cleaning and storage
Cleaning process: Debeard or brush to remove long awns and make the material flowable. Air-screen to clean (See Appendix C for settings).
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Central Iowa Germplasm (IA Zone 2), Northern Iowa Germplasm (IA Zone 1), Southern Iowa Germplasm (IA Zone 3)
Selected Germplasm: Lavaca Germplasm (TX)
Cultivated variety (cultivar): Mandan (ND)
Tested: Icy Blue Germplasm (MI)
- References
Barkworth, M. E., Campbell, J. J.N., & Salomon, B. (2021, May 11). Elymus canadensis L. Flora of North America. http://floranorthamerica.org/Elymus_canadensis
Chayka, K. (n.d.). Elymus canadensis (Canada wild rye). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/canada-wild-rye
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Canada wild-rye. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 264). University of Wisconsin-Madison Arboretum.
Great Plains Flora Association. (1991). Grass family. In T. M. Barkley, R. E. Brooks, & E. K. Schofield (Eds.), Flora of the Great Plains (2nd ed., p. 1167). University Press of Kansas.
Houseal, G. A. (2007). Grasses cool season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 84–85). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Elymus canadensis L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ELCA4
Species Guide Updated 01/31/2025
Virginia wild rye
Virginia wild rye dickeye
Elymus virginicus L.
Alternate Common Name: Terrell grass
Family: grass family (Poaceae)
Functional Group: cool season grasses
Description
- Life cycle and growth form
Perennial, cool season bunchgrass with fibrous roots.
Height: 1-4 ft
- Leaves and stem
Leaf blades mostly flat but sometimes with inrolled edges, rough textured, 12-35 cm (5-14 in) long and 5-15 mm (1/4-5/8 in) wide, sheath sometimes with fine hairs; ligule a short membrane bracketed by two small, often purplish projections (auricles) that may wrap partway around the stem. Stems unbranched, smooth and erect, multiple from the base making clumps.
- Flower, fruit and seedhead
Fruit/seedhead: Seed head is an erect spike 5-17 cm (2-7 in) long (distinguishing it from Canada wildrye which has drooping spikes), often partially enclosed in the uppermost, inflated leaf sheath, light straw-colored at maturity. Spikelets bear awns up to 1 in long. Spikelets eventually drop off, leaving a naked stalk with alternating nodes.
Pollination: wind
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 4,200 (IA NRCS)
Seeds per pound: 67,200 (IA NRCS)
1000 seed weight: 6.14 g (Seed Information Database)
Description: Typical seed unit is a spikelet with 2-3 florets, awned, 0.5-2 cm (1/4-7/8 in) long including awns. Empty scales (glumes) on either side of spikelet thickened, rigid, awned, up to 2.5 cm long (1 in) long including awn. Awn length is highly variable in this species.
Typical seed test
PLS: 96%
Purity: 98%
Germination: 48%
Dormant: 51%
(averages obtained from 9 tests of purchased seed lots)
- Habitat and range
Habitat: Mesic to wet-mesic soil with high fertility; light shade to full sun; deciduous forests, savannas, thickets, meadows near woodlands. Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest. Well-drained loams are preferred for seed production.
Conservation status: Global- G5, secure (NatureServe)

General Comments
Virginia wildrye is commonly found in open forests, savannas, and along woodland edges, and can be particularly abundant in open forests along creeks and rivers. It readily establishes from seed, and holds promise as a nurse crop for prairie and savanna reconstructions. Because of its shade tolerance, it will spread in open woodlands, but eventually gives way to full-sun adapted prairie species in a prairie reconstruction.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in Solid Stand PLS lbs/acre: 8.6 11.5 23 20-35 Seeding depth: 1/4-1/2 in
Seeding method: native seed drill
Seeding time: Fall or early spring
Weed control: Prepare clean, firm, weed free seedbed prior to seeding (e.g., following a glyphosate-resistant crop, for example).
Greenhouse
Seed pre-treatment: No moist stratification is necessary. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed in greenhouse two months before last frost free date at 1/4-1/2 in depth.
Transplanting: Transplant after all danger of frost into rows spaced convenient for tillage equipment or at 12 in spacing in a weed barrier.
- Stand management
Weeds: Mow stands high (6-12 in) first growing season to prevent weed canopy from shading seedlings. Broadleaf herbicides can be used to control broadleaf weeds in established stands. Cultivate or mow between rows.
Pests: Grubs are reportedly a problem in Texas.
Diseases: Ergot is known to occur on seedheads.
Hybridization risk: This species is known to hybridize with related species Elymus canadensis, E. hystrix, E. villosus. Maintain adequate separation between plots of these species.
- Seed production
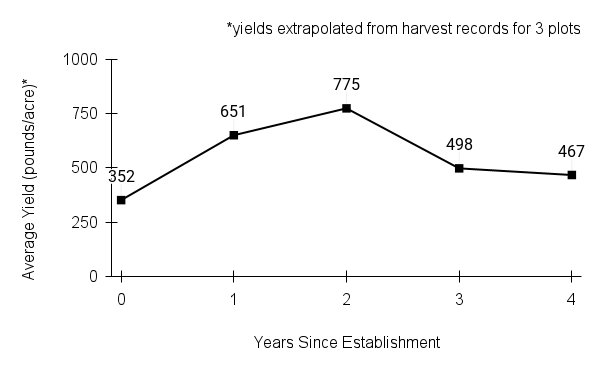 First harvest: Abundant flowering and seed set end of first growing season from greenhouse grown plugs transplanted into a weed barrier.
First harvest: Abundant flowering and seed set end of first growing season from greenhouse grown plugs transplanted into a weed barrier.Yield: 355-775 bulk pounds/acre (per acre yields extrapolated from production on 3 plots)
Stand life: Estimated stand life 5-8 years. Annual early spring burn will prolong the life of the stand.
Flowering date: mid-July to mid-August in northern Iowa
Seed maturity/Harvest date: late August to early September in northern Iowa
Seed retention: Shattering occurs mid to late October; fairly low risk of seed loss.
Harvest date range at TPC (2006-2025): Aug 14 - Nov 22
Harvest date range at Elsberry, MO: Aug 29 to Sept 12
Recommended harvest method: Combine at hard dough stage.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping through 1/2 in mesh to remove large chopped stems and leaves. Debeard or brush gently to remove awns and break up seed heads, then airscreen to clean (see Appendix C for settings).
Seed storage: cool/dry (33-50° F, 30-50% RH).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zones 1, 2, and 3. Northern Missouri Germplasm
Selected Germplasm: Cuivre River Germplasm (MO), Kinchafoonee Germplasm (TX), Tober Germplasm (ND)
- References
Chayka, K. (n.d.). Elymus virginicus (Virginia wild rye). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/virginia-wild-rye
Hilty, J. (2019). Virginia wild rye - Elymus virginicus. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/va_rye.htm
Houseal, G. A. (2007). Grasses cool season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 86–87). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Elymus virginicus L. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ELVI3
Species Guide Updated 12/3/2025
arctic brome
arctic brome dickeye
Bromus kalmii, A. Gray
Alternate Common Names: Kalm’s brome, prairie brome
Scientific Synonyms: Bromopsis kalmii (A. Gray) Holub, Bromus purgans auct. non L, Bromus purgans L., nom. utique rej.
Family: grass family (Poaceae)
Functional Group: cool season grass
Description
- Life cycle and growth form
Short-lived, perennial cool-season grass that grows in distinct tufts or bunches. (In contrast, smooth brome (Bromus inermis) spreads vigorously by rhizomes, and other weedy or invasive brome species are annuals.)
Height: 2 ft

- Leaves and stem

Leaves have flat blades, 3-10 in long and about 3/8 in wide, often hairy, especially near the margins and along the mid-rib; lower sheaths covered in long hairs; flowering stems (culms) are smooth, with short, dense, downward-pointing hairs at the nodes. Note that the leaves of the introduced annual bromes such as cheatgrass are generally narrower than those of arctic brome.
- Flower, fruit and seedhead
Fruit/seed head: Seedhead is a loose panicle that nods gracefully to one side, 4 to 6 in long, bearing spikelets composed of up to 10 florets that are densely hairy and have short awns (2-3 mm). The easily visible hairiness of the spikelets help to distinguish this species from smooth brome and the shorter awns differentiate it from other weedy or invasive members of the genus.
Pollination: wind
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 8,000 seeds (IA NRCS)
1000 seed weight: 2.55g (Seed Information Database)
Description: Grains are strongly flattened and ovoid, 6-8 mm long, with a bundle of white hairs at the tip, enclosed in hairy bracts (lemma and palea)
Typical seed test
PLS: 85%
Purity: 90%
Germination: 25%
Dormancy: 61%
(averages obtained from 11 tests of purchased seed lots)
- Habitat and range
Habitat: Dry to moist soil; partial to full sun; prairies, prairie remnants, meadows, fens, savannas, open woodlands; Wetland Indicator Status is Facultative (FAC) for the Midwest; mesic to dry well-drained loamy soils are recommended for seed production.
Conservation status: Global- G5, secure; District of Columbia, Maryland, and New Hampshire- SH, possibly extirpated; Maine and Virginia- S1, critically imperiled; New Jersey, Ohio, Pennsylvania, and Vermont- S2, imperiled; Illinois- S2/S3, imperiled to vulnerable; Iowa and North Dakota- S3, vulnerable (NatureServe)
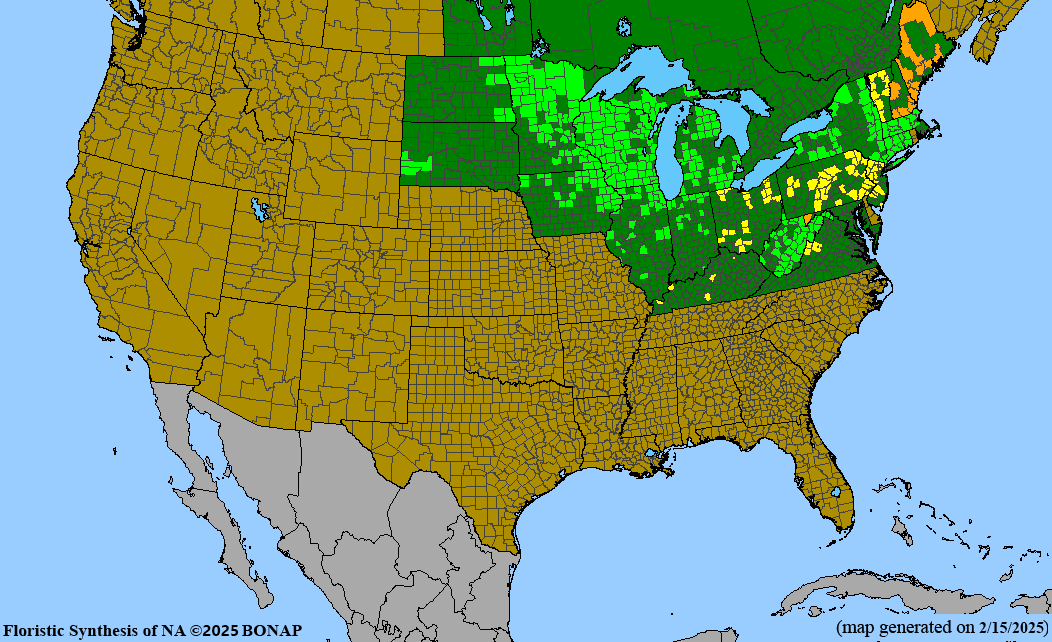
General Comments
The name “brome” is not one that most prairie restoration practitioners hope to see in plant lists due to their familiarity with the highly competitive, invasive, sod-forming species, smooth brome. However, native species in the genus Bromus such as Bromus kalmii (called “arctic brome” using USDA Plants nomenclature or more commonly “prairie brome” or “Kalm’s brome”) are valuable additions to restoration seed mixes in the Upper Midwest. Arctic brome is distinguishable from smooth brome by its clumping, nonrhizomatous growth habit and the preponderance of soft hairs on its leaf sheaths, leaves (often), stem nodes, and seedheads. Plantings by the Tallgrass Prairie Center have included this grass since at least 2016, and monitoring shows that it establishes and persists in planted prairies in our area and coexists alongside other grasses and forbs. Iowa Source Identified arctic brome provides regionally appropriate material for another option to fill the cool season graminoid component of seed mixes. This is also an elegant and shorter statured native grass for landscape design applications.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Direct seeding methods shared by a commercial native seed grower
Seeding rate: 15 pounds/acre
Row spacing: solid stand
Seeding depth: surface
Seeding method: broadcast
Seeding time: dormant season
Weed control: Prepare clean, firm, weed free seedbed prior to seeding (e.g., following a glyphosate-resistant crop, for example).
Greenhouse
Seed pre-treatment: Cold/moist stratification for 30 days produced even germination.
Sowing: Lightly cover seed in germination flats or plugs in the greenhouse about 8-12 weeks before average frost free date. Germination begins about one week after sowing.
Transplanting: When plugs are well-rooted, move them outside to harden off for a week or two, then transplant into plastic mulch with 8-12 in spacing between plants after danger of frost is past.
- Stand management
Weeds: If direct seeded, mow stands high (6-12 in) during the first growing season to prevent weed canopy from shading seedlings. We do not currently have information on herbicides that could be used for weed control in this crop. Cultivate or mow between rows. Hand rogue before harvest to remove potential weed seed contaminants.
Pests: None noted.
Diseases: None noted.
- Seed production
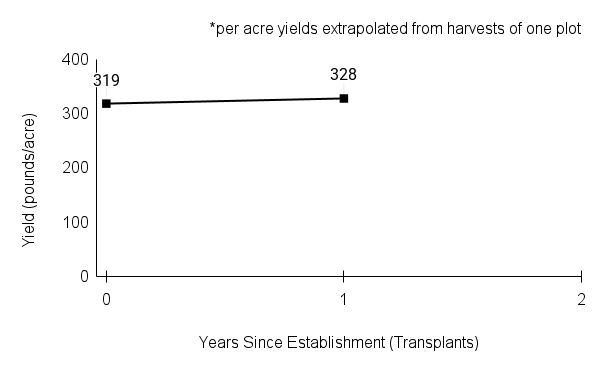 First harvest: Flowering and seed set in first growing season from transplants; probably in the second year from direct seeding.
First harvest: Flowering and seed set in first growing season from transplants; probably in the second year from direct seeding.Yield/acre: About 320 pounds/acre (extrapolated from two years of harvests of one production plot).
Stand life: Unknown at this time but likely 3-5 years. Invasion of plot by non-native cool season grasses (quack grass and smooth brome) seems to be the greatest challenge.
Flowering date: June to July in northeast Iowa
Seed maturity/harvest date: mid to late July in northeast Iowa (first year harvests from transplants are delayed into September)
Seed retention: Seed is relatively resistant to shattering, though high winds and rain can cause some loss of seed. Frequent monitoring recommended as seed matures. In remnant prairies, we observed that some seed remained on plants well into September.
Harvest date range at TPC (2024 - 2025): July 14 to Sept 3 (September date was in the first growing season)
Recommended harvest method: combine
- Seed cleaning and storage
Cleaning process: Scalp material through 1/2 in mesh if needed to remove larger debris. Brush material to make it more flowable, then airscreen. Indent, if needed, to remove shorter seeded weeds.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone EA (eastern Iowa)
- References
Chayka, K. (n.d.). Bromus kalmii (Kalm’s brome). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/kalms-brome
Hilty, J. (2019). Prairie brome - Bromus kalmii. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/pr_brome.htm
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
Pavlickf, L. E. (2021, May 11). Bromus kalmii A. Gray. Flora of North America. http://floranorthamerica.org/Bromus_kalmii
USDA NRCS National Plant Data Team. (n.d.). Bromus kalmii A. Gray. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=BRKA2
Species Guide Updated 12/17/2025
bluejoint
bluejoint dickeye
Calamagrostis canadensis (Michx) P. Beauv.
Alternate Common Names: bluejoint reedgrass, Canada bluejoint, Canada reedgrass, marsh reedgrass, meadow pinegrass
Scientific Synonym: Calamagrostis anomala
Family: grass family (Poaceae)
Functional Group: cool season grasses
Description
- Life cycle and growth form
Perennial, rhizomatous grass that forms spreading, hummocky colonies.
Height: Flowering culms 3-5 ft tall

- Leaves and stem
Leaf blades up to 12 in (30 cm) long and 3/16-3/8 in (3-8 mm) wide, leaf sheaths smooth with prominent veins; ligule is membranous, about 1/8 in (3 mm) tall; stems and nodes are hairless; dead leaves and stems accumulate from year to year in absence of fire, forming tussocks.
- Flower, fruit and seedhead
Fruit/seedhead: Mature seed head is a pale gold, loosely branched, plume-like panicle 4-8 in long (10-20 cm), tending to nod to one side; short “flag leaf” just below panicle tends to stick out at a 90 degree angle from the stem; florets fall off when mature, leaving glumes on the stalks.
Pollination: Wind
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 280,000 (IA NRCS)
Seeds per pound: 4,480,000 (IA NRCS)
1000 seed weight: 0.32 g (Seed Information Database)
Description: Spikelets about 2 mm (3/32 in) long. Grain (caryopsis) about 1/32-1/16 in (1 mm) long, with tuft of hairs at the base slightly shorter than the grain.
Typical seed test
PLS: 90%
Purity: 95%
Germination: 73%
Dormant: 22%
(averages obtained from 4 tests of purchased seed lots)
- Habitat and range
Habitat: Wet-mesic to wet soil; full sun; bogs, marshes, wet swales, along rivers and streams. Tolerates acidic soils up to pH 8 conditions; can tolerate anaerobic (low oxygen) conditions; prefers nutrient-rich, seasonally-inundated soils; irrigation is essential for optimal seed production on upland sites. Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest.
Conservation status: Global- G5, secure; North Carolina, Delaware, and Kansas- S1, critically imperiled; Illinois- S3, vulnerable (NatureServe)
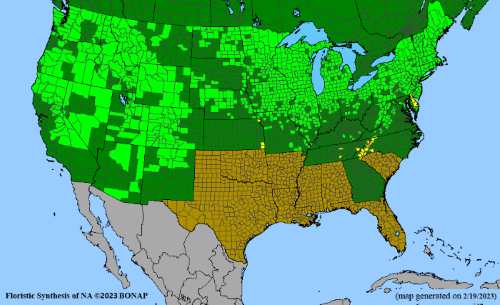
General Comments
Bluejoint is a highly rhizomatous species forming large colonies in preferred habitats, occupying sites even more wet than prairie cordgrass (Spartina pectinata) seems to prefer. Like prairie cordgrass, seed production occurs mostly on the outer edges of colonies, and is generally low. Bluejoint is best propagated in controlled conditions of the greenhouse, and transplanted into wide row spacings.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species
Greenhouse
Seed pre-treatment: No stratification necessary. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed on surface of growing mix in greenhouse two months before last frost free date. Water carefully with a mist wand to avoid dislodging seed.
Transplanting: Transplant after all danger of frost. Greenhouse grown plugs can be transplanted into wide row spacing, 6-8 ft between rows, and plants should be 1-2 ft apart within the rows. This gives the newly established plants adequate root-space for rhizome spread, and promotes more flowering and seed set after establishment. Irrigate during establishment and as needed for flowering and seed production.
- Stand management
Weeds: Pre-emergent herbicides can be used after transplanting seedling plugs or pieces of rhizome. It’s critical to water-in transplants to seal soil around roots to prevent herbicide from coming into contact with and possibly damaging roots.
Pests: Nematode (Subanguina calamagrostis) invades leaf tissue and form galls, causing leaves to twist, and allowing subsequent infection by a fungus (Norton 1987).
Diseases: None noted.
- Seed production
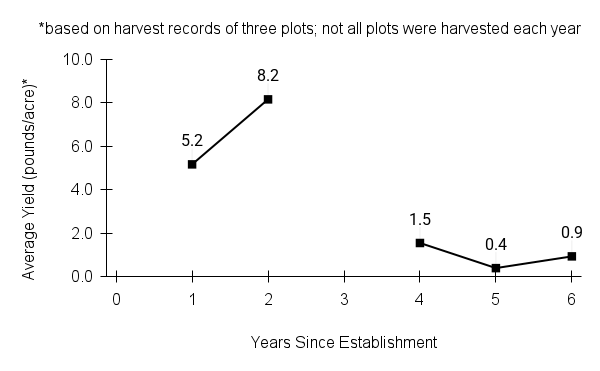 First harvest: Plants remain vegetative first growing season. Some flowering and seed set in the second growing season from greenhouse grown transplants.
First harvest: Plants remain vegetative first growing season. Some flowering and seed set in the second growing season from greenhouse grown transplants.Yield: 0.4-8.2 bulk pounds/acre (yields extrapolated based on production from 3 plots); commercial growers can achieve yields of 20-50 pounds per acre (USDA Plants Guide)
Stand life: Stands are long-lived in proper soils/hydrology. Seed production declines as stands become sod-bound in approximately 4-5 years. Flowering is patchy within a stand and may be higher on the edges of clones.
Flowering date: mid to late June in northern Iowa
Seed maturity: early July in northern Iowa
Seed retention: Shattering occurs soon after maturity, and seed is windblown. Monitor frequently for ripeness and be ready to harvest when inflorescences turn pale, fluffy, and open. The glumes stay on the plant when the seed drops, so seed heads can appear full even after they have shed much of their seed.
Harvest date range at TPC (2003-2008): June 29 - July 10
Recommended harvest method: Hand harvest or combine at maturity, but before dispersal. The seed is very light and wind-dispersed. Turn off air when combining.
- Seed cleaning and storage
Cleaning process: Thresh hand-collected material through 1/4 in screen. Brush seed to remove tuft of hairs at base of florets to improve flow of seed through airscreen (see settings in Appendix C).
Seed storage: Cool/dry (33-50° F, 30-50% RH).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zones 1, 2, and 3
Cultivated variety (cultivars): Sourdough (AK)
- References
Alaska DNR. (2007). ‘Sourdough’ Bluejoint Reedgrass Calamagrosis canadensis. [Infographic]. dnr.alaska.gov. https://dnr.alaska.gov/ag/akpmc/pdf/plant-flyers/SourdoughBluejoint.pdf
Chayka, K. (n.d.). Calamagrostis canadensis (Canada bluejoint). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/canada-bluejoint
Hilty, J. (2019). Bluejoint grass - Calamagrostis canadensis. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/bluejoint.html
Houseal, G. A. (2007). Grasses cool season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 82–83). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Marr, K. L., Hebda, R. J., & Greenef, C. W. (2021). Calamagrostis canadensis (Michx.) P. Beauv. Flora of North America. http://floranorthamerica.org/Calamagrostis_canadensis
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Norton, D.C., Cody, A.M., Gabel, A.W. (1987) Subanguina calamagrostis and Its Biology in Calamagrostis spp. in Iowa, Ohio, and Wisconsin. The Journal of Nematology, 19(2), 260-262. https://pmc.ncbi.nlm.nih.gov/articles/PMC2618621/
USDA NRCS National Plant Data Team. (n.d.). Calamagrostis canadensis (Michx.) P. Beauv. USDA plants database. https://plants.usda.gov/plant-profile/CACA4
Wynia, R.L. (2006, March 07) Bluejoint Reedgrass Calamagrostis canadensis (Michx.) Beaux. USDA NRCS Plant Guide. https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_caca4.pdf
Species Guide Updated 12/1/2025
hairy wildrye
hairy wildrye dickeye
Elymus villosus, Muhl. ex Willd
Alternate Common Names: silky wildrye, downy wildrye
Scientific Synonyms: Elymus arkansanus Scribn. & C.R. Ball, Elymus canadensis L. var. Villosus (Muhl. ex Willd.) Shinners, Elymus villosus Muhl. ex Willd. var. Arkansanus (Scribn. & C.R. Ball) J.J.N. Campbell, Terrellia villosa (Muhl. ex Willd.) Baum
Family: grass family (Poaceae)
Functional Group: cool season grass
Description
- Life cycle and growth form
Perennial, cool season grass from fibrous roots (no rhizomes), forming leafy tufts.
Height: 2.5 - 3.5 ft
- Leaves and stem

Leaves flat, dark green with a soft texture from their abundant short, silky hairs; sheath is often hairy; two small purplish projections at the collar (auricles) wrap partly to all the way around the stem.
- Flower, fruit and seedhead
Fruit/seedhead: Inflorescence a single, nodding spike per culm, 1.5 - 5 in long, with many spikelets, bristly due to long awns (1 to 1 1/2 in long) on glumes and lemmas; glumes and lemma (one of the pair of bracts around the grain) are finely hairy.
Pollination: wind
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 5,500 seeds/oz (IA NRCS)
1000 seed weight: 3.30g (Seed Information Database)
Description: Typical seed unit consists of sterile bracts (lemma and palea) enclosing the elliptical grain (1.5 mm by 5-6 mm).
Typical seed test
PLS: 91.6%
Purity: 98.5%
Germination: 89%
Dormant: 4%
(Values obtained from 1 test of a seed lot produced at TPC; note that this seed lot was 11 years old at time of test)
- Habitat and range
Habitat: Dry, sandy or gravelly soil; part shade to full sun; woods, floodplain forests, river banks, ravines, wooded slopes, rock outcrops; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest; moist, well-drained, loamy soils are recommended for seed production.
Conservation status: Global- G5, secure; Massachusetts, Rhode Island, and Vermont- S1, critically imperiled; Mississippi and North Carolina- S2, imperiled; Wyoming- S2/S3, imperiled to vulnerable; Delaware- S3, vulnerable (NatureServe)
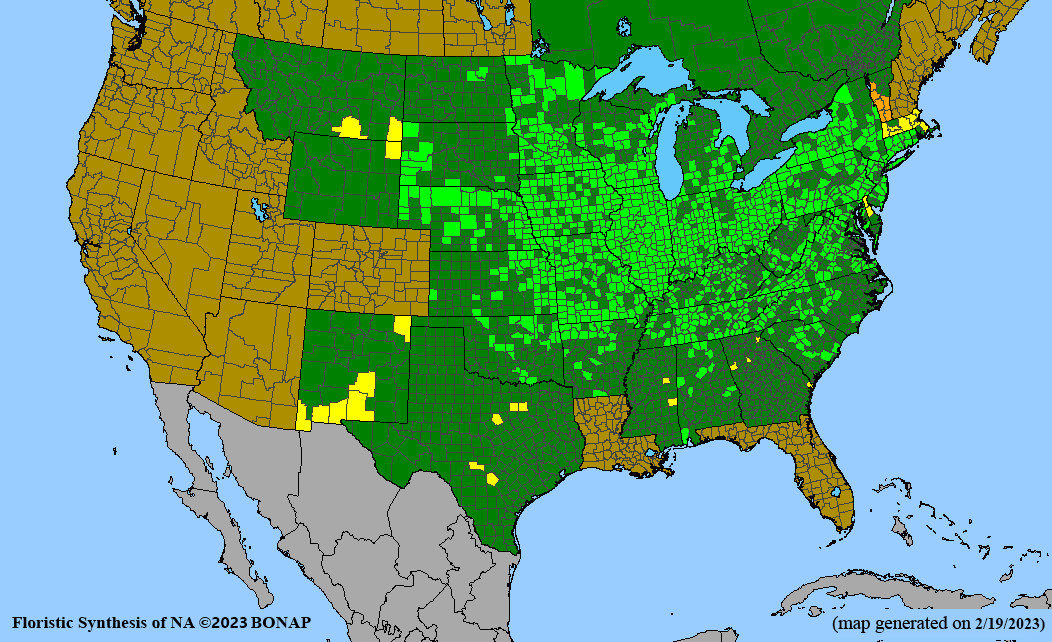
General Comments
Silky wildrye is typically found in wooded areas, but it tolerates full sun in production areas as long as the site is not excessively dry. This species somewhat resembles Canada wildrye (Elymus canadensis) but has straight awns in mature seedheads, slender glumes, fine hairs within the inflorescences, and generally more hairy leaves and sheaths.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Direct seeding methods shared by a commercial native seed grower.
Row spacing: Solid stand
PLS pounds/acre: 12
Seeding depth: Surface (1/4 to 1/2 in depth with native seed drill should also be effective)
Seeding method: broadcast
Seeding time: late fall or early spring
Weed control: Prepare clean, firm, weed free seedbed prior to seeding (e.g., following a glyphosate-resistant crop, for example).
Greenhouse
Seed pre-treatment: 30 day cold/moist stratification produces rapid, even germination
Sowing: Sow seed in germination flats or plugs lightly covered (1/8 - 1/4 in) with potting mix about 2 months before the last frost free date.
Transplanting: Harden off plugs for a week or two outside, then transplant after all danger of frost into rows spaced convenient for tillage equipment or at 8-12 in spacing in a weed barrier or plastic mulch.
- Stand management
Weeds: Mow direct seeded stands high (6-12 in) first growing season to prevent weed canopy from shading seedlings. We do not currently have information on herbicides that could be used for weed control in this crop. Cultivate or mow between rows and weed or hand rogue to prevent contamination of seed lots by weed seed.
Pests: None noted.
Diseases: None noted.
Hybridization risk: This species is known to hybridize with related species Elymus canadensis, E. hystrix, E. virginicus. Maintain adequate separation between plots of these species.
- Seed production
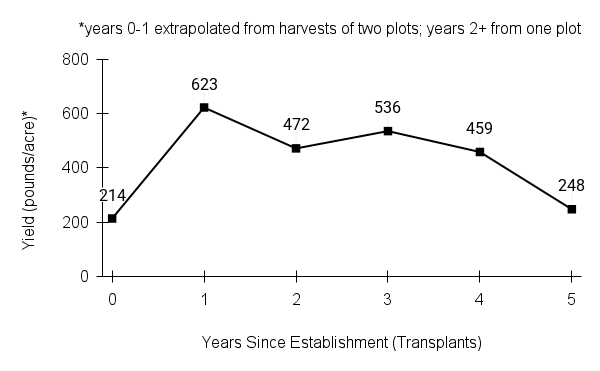 First harvest: Some flowering and seed set in the establishment year when transplanted as plugs. First harvest in second growing season from direct seeding.
First harvest: Some flowering and seed set in the establishment year when transplanted as plugs. First harvest in second growing season from direct seeding.Yield/acre: 200 - 620 pounds/acre (extrapolated from harvests of two plots at TPC)
Stand life: Five years or more, though production may begin to decline by years 4-5.
Flowering date: June in northeast Iowa
Seed maturity/Harvest date: Late July - Aug in northeast Iowa
Seed retention: Moderate risk of shattering; spikelets begin to drop in August.
Harvest date range at TPC (2010-2025): July 27 - August 30 (early September harvest is possible in first season from transplants)
Recommended harvest method: Combine at hard dough stage (no clogging observed despite awns)
- Seed cleaning and storage
Cleaning process: Pass combined material through 1/2 in mesh to remove large particles. Brush to remove awns and make flowable, then airscreen.
Seed storage: Seed may retain viability for 10 years or more when stored in cool/dry conditions (33-50° F, 30-50% RH).
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone “Iowa”
- References
Barworth, M. E., Campbell, J. J. N., & Salomon B. (2021, May 11). Elymus villosus Muhl. ex Willd. Flora of North America. http://floranorthamerica.org/Elymus_villosus
Chayka, K. (n.d.). Elymus villosus (silky wild rye). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/silky-wild-rye
Great Plains Flora Association. (1991). Grass family. In T. M. Barkley, R. E. Brooks, & E. K. Schofield (Eds.), Flora of the Great Plains (2nd ed., p. 1169–1170). University Press of Kansas.
Hilty, J. (2019). Silky wild rye - Elymus villosus. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/silky_rye.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Elymus villosus Muhl. ex Willd. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=ELVI
Species Guide Updated 12/18/2025
prairie Junegrass
prairie Junegrass dickeye
Koeleria macrantha (Ladeb.) Schult.
Alternate Common Names: June grass, Junegrass, crested hair grass, Koeler’s grass
Scientific Synonyms: Koeleria albescens auct., Koeleria cristata auct. non Pers. p.p., Koeleria cristata Pers. var. longifolia Vasey ex Burtt Davy, Koeleria cristata Pers. var. pinetorum Abrams, Koeleria gracilis Pers., Koeleria nitida Nutt., nom. utique rej., Koeleria pyramidata auct. non (Lam.) P. Beauv. p.p., Koeleria yukonensis Hultén, Koeleria nitita, Koeleria cristata Pers. var. pubescens, Koeleria cristata Pers. var. major
Family: grass family (Poaceae)
Functional Group: cool season grasses
Description
- Life cycle and growth form
Perennial, cool-season bunchgrass with fibrous roots.
Height: 0.5-2 ft

- Leaves and stem

Leaves mostly basal in a distinct tuft, leaf blades 3-25 cm long (1.25-10 in) and 1-4 mm wide (up to 3/16 in), leaf sheaths short-hairy to smooth, with hairs on the margins of the collar, ligule a short (<1 mm), ragged membrane; stem with fine hairs at base of seedhead and at nodes.
- Flower, fruit and seedhead
Fruit/seedhead: Light tan spike-like panicle 3-18 cm long (1.25-7.25 in) and 1-3 cm wide (0.5-1.25 in), stem covered in fine hairs below the seed head. At maturity, seed drops off with surrounding chaffy parts (lemma and palea), but glumes (chaffy parts below the flowers) stay on the stalk.
Pollination: wind
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 200,000 (IA NRCS)
Seeds per pound: 3,200,000 (IA NRCS)
1000 seed weight: 0.30 g (Seed Information Database)
Description: Seed unit is a grain about 2-3 mm long (1/8 in), with or without surrounding chaffy parts (lemma and palea).
Typical seed test
PLS: 69% (n = 10)
Purity: 76% (n = 10)
Germination: 68% (n = 9)
Dormant: 1% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Full sun. Found in dry, upland, rocky or sandy prairies, becoming more abundant on northern prairies. Very well-drained soils are preferred for seed production.
Conservation status: Global- G5, secure; Pennsylvania- SX, presumed extirpated; Alaska, Kentucky, and Louisiana- S1, critically imperiled; Arkansas and Ohio- S2, imperiled; Nevada- S3, vulnerable (NatureServe)

General Comments
Prairie Junegrass is an important cool-season grass component, particularly in prairies on dry, very well-drained sites. Plants are of medium longevity, poorly tolerant of competition, and may rely on reseeding to persist.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Row spacing: 36 in 24 in 12 in Solid Stand PLS lbs/acre: 0.25 0.4 0.75 0.75-1.0 Seeding depth: 1/8 in (light needed for germination)
Seeding method: native seed drill
Seeding time: Late spring
Weed control: Good weed control before seeding is essential. Seedlings are small and slow growing and vulnerable to competition from weeds as well as to mechanical damage from equipment or foot traffic.
Greenhouse
Seed pre-treatment: No stratification necessary, although we have observed improved speed and uniformity of germination following 30-day cold/moist stratification. Germination of grass seed usually improves with proper storage (cool, dry conditions) throughout the first year after harvest.
Sowing: Sow seed on top of soil (or cover only very lightly, seeds require light to germinate) in a greenhouse 8-12 weeks before the last frost free date. Water (or mist) very gently to avoid splattering or floating seeds out of the potting medium.
Transplanting: Transplant into a weed barrier 8-12 in apart after all danger of frost.
- Stand management
Weeds: For plantings in weed barrier, hand rogue weeds while small, being careful not to uproot young seedlings. For direct seeded stands, mow stand high (6–12 inches) in first growing season to prevent weed canopy from shading seedlings. Cultivate or mow vegetation between rows. Broadleaf herbicides can be used to control broadleaf weeds in established stands. Non-native perennial cool-season grasses such as Kentucky bluegrass (Poa pratensis) and smooth brome (Bromus inermis) invade plantings and can outcompete Junegrass, causing declines in production.
Pests: Grubs are reportedly a problem in Texas.
Diseases: Ergot is known to occur on seedheads.
- Seed production
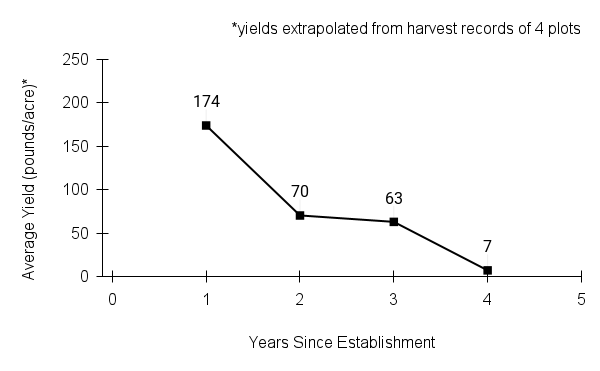 First Harvest: Flowering and seed set at end of second growing season from greenhouse grown transplants. Plants will remain vegetative the first growing season.
First Harvest: Flowering and seed set at end of second growing season from greenhouse grown transplants. Plants will remain vegetative the first growing season. Yield: 10-175 pounds/acre (averages extrapolated from 3 plots)
Stand life: Potentially 4-5 years. Peak harvests occur in second and third year, after which seed production declines significantly. Invasion by non-native cool-season grasses is one cause of yield decline.
Flowering Date: early to late June in northern Iowa
Seed maturity/Harvest date: late June - early July in northern Iowa. Regularly monitor plots for maturity as seed heads change from green to straw colored. Tap seed heads lightly on hand and observe for shattering.
Seed retention: Shattering occurs mid to late July
Harvest date range at TPC (2003-2023): June 22 - July 8
Recommended harvest method: Combine harvesting is practical for larger plots. Swathing/windrowing before combining may improve threshing, uniformity of seed maturation, and reduce shattering due to wind. A modified hedge-trimmer with an attached collection tray works for harvesting small plots.
- Seed cleaning and storage
Cleaning process: Pre-clean air-dried material by scalping thru 1/2 in and 1/4 in mesh to remove large pieces of stems and leaves. Run through a brush machine to break up seed heads, then air-screen to clean (see appendix for settings).
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Project Zone 1 (northern Iowa) and Zone 2 (central Iowa)
- References
Chayka, K. (n.d.). Koeleria macrantha (Junegrass). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/junegrass
Hilty, J. (2019). June grass - Koeleria macrantha. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/june_grass.htm
Houseal, G. A. (2007). Grasses cool season. In G. A. Houseal (Eds.), Tallgrass Prairie Center’s native seed production manual (pp. 88–89). Tallgrass Prairie Center - University of Northern Iowa.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Runkel, S. T., & Roosa, D. M. (2009). June grass. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 106–107). University of Iowa Press.
Standley, L. A. (2021, May 11). Koeleria macrantha (Ledeb.) Schult. Flora of North America. http://floranorthamerica.org/Koeleria_macrantha
USDA NRCS National Plant Data Team. (n.d.). Koeleria macrantha (Ledab.) Schult. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=KOMA
Species Guide Updated 12/04/2025
Woody Species
Woody Species sagemThe Species Production Guides for woody species (prairie shrubs) provide specific information about growing each of these species for seed production.
A printable file (pdf) is also provided on each species page for those needing a print version.
This section is a work in progress. We will continue to add new species guides as they are completed.
Rosa arkansana / prairie rose
- Spiraea alba / white meadowsweet
false indigo bush
false indigo bush sagem
Amorpha fruticosa L.
Alternate Common Names: false indigo, bastard indigo, river locust, wild indigo, indigo bush, desert false indigo
Scientific Synonyms: Amorpha angustifolia (Pursh) Boynt., Amorpha bushii Rydb., Amorpha croceolanata P.W. Watson, Amorpha curtissii Rydb., Amorpha dewinkeleri Small, Amorpha occidentalis Abrams, Amorpha tennesseensis Shuttlw. ex Kunze, Amorpha virgata Small
Family: legume or pea family (Fabaceae (Leguminosae))
Functional Group: woody species, shrubs
Description
- Life cycle and growth form
Fast-growing, long-lived perennial shrub, spreads by self seeding and suckering, flowers on second-year wood.
Height: 3 -12 ft

- Leaves and stem

Leaves 4-8 in long, pinnately compound with 11-25 oblong leaflets, alternate arrangement; multi-stemmed shrubs with smooth, gray, woody stems, forming thickets of spreading, cane-like stems that begin sparsely branching at about 3-4 ft in height.
- Flower, fruit and seedhead
Flower: Irregular, with one slightly enlarged petal (unlike the typical legume flower), deep purple corolla 1/3 in long, with bright orange stamens that stick out prominently; flowers tightly packed in spikelike racemes 3-6 in long (each looking like a tapered bottle brush).
Fruit/seedhead: 1/4 in long, tough, leathery seed pods with prominent oil glands, each pod with one seed (sometimes two).
Pollination: Insects, primarily bees.

- Seed

Note: Seed produced from unirrigated rows at the TPC had much lower viability (PLS: 16-23%, not included in the typical seed test above).
Seed characteristics
Seeds per ounce: 3,700 (IA NRCS)
Seeds per pound: 77,000 (Woody Plant Seed Manual)
1000 seed weight: 7.76 g (Seed Information Database)
Description: Glossy, light-brown seed resembles a small bean, 4 mm long and 1.5 mm wide, with a slightly hooked end.
Typical seed test
PLS: 90-94%
Purity: 98%
Germination: 31-92%
Dormant: 0-65%
(based on tests of one lot of commercial seed and one lot produced at the TPC)
- Habitat and range
Habitat: Moist soil; partial to full sun; along river and stream banks, islands, ditches, wet prairies, and seeps. Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest. Plants survive for many years in mesic soils without irrigation, but seed yield (and possibly viability) increases with irrigation.
Conservation status: Global- G5, secure; Wyoming- S2, imperiled; West Virginia- S2/S3, imperiled to vulnerable (NatureServe); Listed as a noxious weed or invasive plant in Maine, Rhode Island, New Jersey, New Hampshire, Oregon, and Washington.
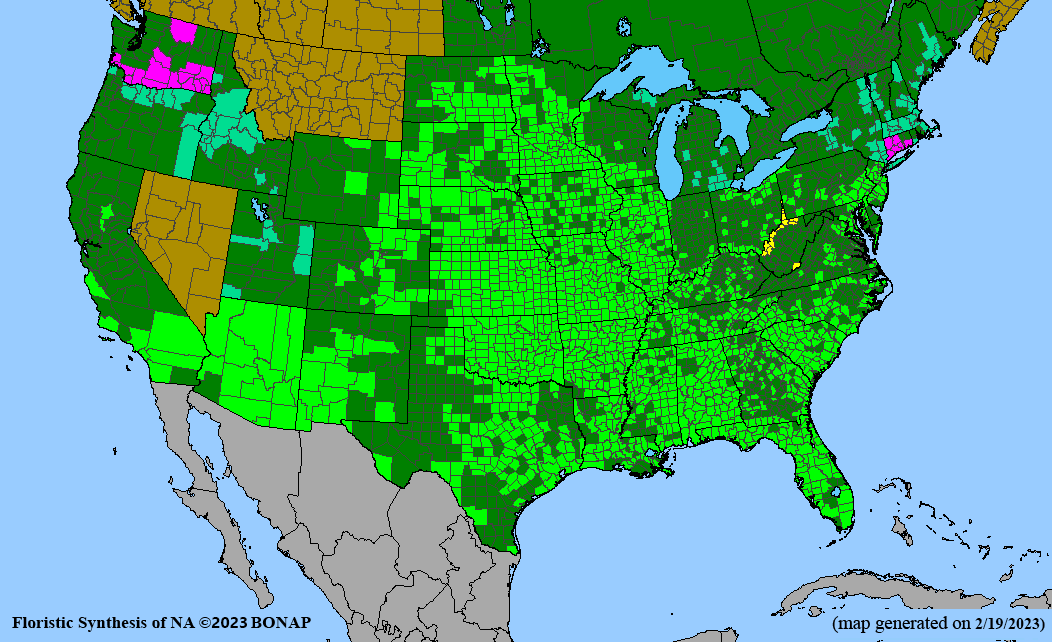
General Comments
The dark purple flower spikes with brilliant orange pollen-bearing stamens attract numerous species of native bees in great abundance, along with skippers, other butterflies, and moths. The foliage and pods, when crushed, have an unusual scent, reminiscent of cumin, citrus, and creosote. Aromatic compounds from this species have been investigated as medicines, natural insecticides and insect repellents. The foliage is eaten by larvae of silver-spotted skipper and southern dogface butterflies, larvae of amorpha borer beetles live within the stems and roots, and tiny bruchid beetles feed on the seeds. The long, weakly branched stems have been used in arrow-making and as a foundation for bedding materials by Native peoples. The functions this species provides in restoration include erosion control, streambank stabilization, wildlife cover, and windbreaks, and it shows potential for use in living snow fences.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production. Widely spaced rows (6 ft or more) are recommended.
Greenhouse
Seed pre-treatment: Start seed pretreatment about 3-4 months before outplanting. Mechanical or chemical scarification is recommended as these seeds have physical dormancy, followed by 10-14 days cold/moist stratification (40°F).
Sowing: Cover seed lightly (1/4 in depth) with potting mix; adding a layer of perlite or chick grit to the surface of the soil may help prevent damping off.
Transplanting: Seedlings are ready for transplanting when roots have reached the bottom of the plug and are well-branched, creating a firm plug. Harden off outdoors, then transplant into plasticulture rows with drip irrigation, 2-3 plants per linear foot.
- Stand management
Weeds: Plastic mulch controls weeds in year one. Shrubs grow vigorously and shade out most weeds.
Pests: In very snowy winters, rabbits feed on bark and may girdle stems. Shrubs will resprout but flowering and seed set will be delayed for a year. Deer occasionally browse the tops of plants. Some native insects feed on foliage or within stems/roots, but not at densities that cause production issues. Bruchid beetles feed on developing seeds within pods and can reach significant densities. However, seed yield is still high in “good years” and when rows are irrigated.
Diseases: An unidentified rust fungus causes leaves to appear distorted in some years.
Note: Rows can be cut back to the ground in the dormant season if plants have become too lanky for efficient harvesting. Note that this shrub species does not flower or set seed on first year growth.
- Seed production
 First harvest: Plants flower and set seed one year after transplanting.
First harvest: Plants flower and set seed one year after transplanting.Yield: 80-580 pounds/acre (based on 3 plots)
Stand life: More than 10 years (estimated) for these long-lived shrubs.
Flowering date: June
Seed maturity/Harvest date: October
Seed retention: Low risk of shattering; pods remain on plants through late October.
Harvest date range at TPC (2018-2021): Aug 30 - Oct 31
Recommended harvest method: Strip pods from stalks by hand (wear sturdy gloves). We have not attempted to combine this species due to the woody stems. A more efficient approach might be to cut the fruiting stems onto tarps using a hedge trimmer, then run the material through a stationary combine.
- Seed cleaning and storage
Cleaning process: Pass hand-collected pods through a coarse screen (1/4 to 1/2 inch hardware cloth) to remove sticks. Run through a brush machine with canvas beater bars. Seed pods have oil glands (visible under low magnification) and become very sticky when brushed. Spread oily material on a tarp and dry with a fan for a few days. Material may need to be brushed a second time after drying and before airscreening.
Seed storage: cool/dry (33-50° F, 30-50% RH) - seed retains viability for 3-5 years at room temperature (Woody Plant Seed Manual).
Released Germplasm
Source identified material: Natural Selections/Iowa Ecotype Zones 1, 2, 3
Selected germplasm: Iowa Covey Germplasm, Illinois Covey Germplasm, Missouri Covey Germplasm
Tested germplasm: Survivor Germplasm (ID)
- References
Bonner, F. T., Karrfalt, R. P., & Nisley, R. G. (Eds.). (2008). Woody plant seed manual. RNGR - Reforestation, Nurseries, and Genetic Resources. https://rngr.net/publications/wpsm
Hilty, J. (2019). False indigo - Amorpha fruticosa. Illinois Wildflowers. https://www.illinoiswildflowers.info/trees/plants/false_indigo.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Lady Bird Johnson Wildflower Center - The University of Texas at Austin. (2022). Amorpha fruticosa. Plant Database. https://www.wildflower.org/plants/result.php?id_plant=amfr
Moore, L. M. (2006). Plant guide: Desert false indigo - Amorpha fruticosa L. USDA-NRCS National Plant Data Center. https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_amfr.pdf
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 22, 2024).
Newcomb, L. (1977). Shrubs / leaves divided. In Newcomb’s wildflower guide (pp. 106–107). Little, Brown and Company.
Runkel, S. T., & Roosa, D. M. (2009). Indigo bush. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 66–67). University of Iowa Press.
University of Georgia - Center for Invasive Species and Ecosystem Health. (n.d.). Indigobush (Amorpha fruticosa). EDDMapS. https://www.eddmaps.org/species/subject.cfm?sub=5086
USDA NRCS National Plant Data Team. (n.d.). Amorpha fruticosa L. USDA plants database. https://plants.usda.gov/plant-profile/AMFR
Species Guide Updated 12/18/2024
white meadowsweet
white meadowsweet sagem
Spiraea alba Du Roi
Alternate Common Names: meadow sweet, meadowsweet, narrow-leaved meadowsweet, American meadowsweet, pale bridewort, pipestem, queen of the meadows
Family: rose family (Rosaceae)
Functional Group: woody species, shrubs
Description
- Life cycle and growth form
Perennial shrub with woody root system, growing in colonies of slender stems.
Height: 2-4 ft

- Leaves and stem

Leaves are alternate, mostly hairless, narrowly elliptic, 2-3 in long and 3/4 in wide, with finely serrate margins and short petioles; stems are smooth, slender, and woody, with few branches, becoming brown with age, multiple stems produced from the same rootstock.
- Flower, fruit and seedhead
Flower: Radially symmetrical, 1/4 in wide flowers are five-parted with white petals, a pink, yellow, or orange center ring, and long stamens that stick out from the flowers; inflorescence is a branched cluster of spikes 2 - 6 in long, each with numerous flowers, blooming from the top down.
Fruit/seedhead: Each flower forms four to six (usually five) dry, reddish-brown fruits (follicles), arrayed in a star-like cluster; each follicle is tough, short-beaked, hairless, and contains 2-5 seeds; ripe follicles split open along one side to release the seeds.
Pollination: insects, particularly bees

- Seed
Seed characteristics
Seeds per ounce: 300,000 (Prairie Moon)
1000 seed weight: 0.88 g (Seed Information Database)
Description: Slender, banana-shaped seeds are 2 mm long by less than 0.5 mm wide and a rusty orange color.
Typical seed test
TZ-PLS: 53%
Purity: 60%
TZ: 88%
(averages obtained from 3 seed lots produced at TPC)
- Habitat and range
Habitat: Grows in moist to wet soil in full sun; found in wet prairies, along streams, bogs, marsh edges, ditches; Facultative Wetland status in Midwest (USDA Plants Database); benefits from irrigation in seed production systems.
Conservation status: Global- G5, secure; Delaware and Tennessee- S1, critically imperiled; North Carolina- S2, imperiled; South Dakota- S3, vulnerable (NatureServe)
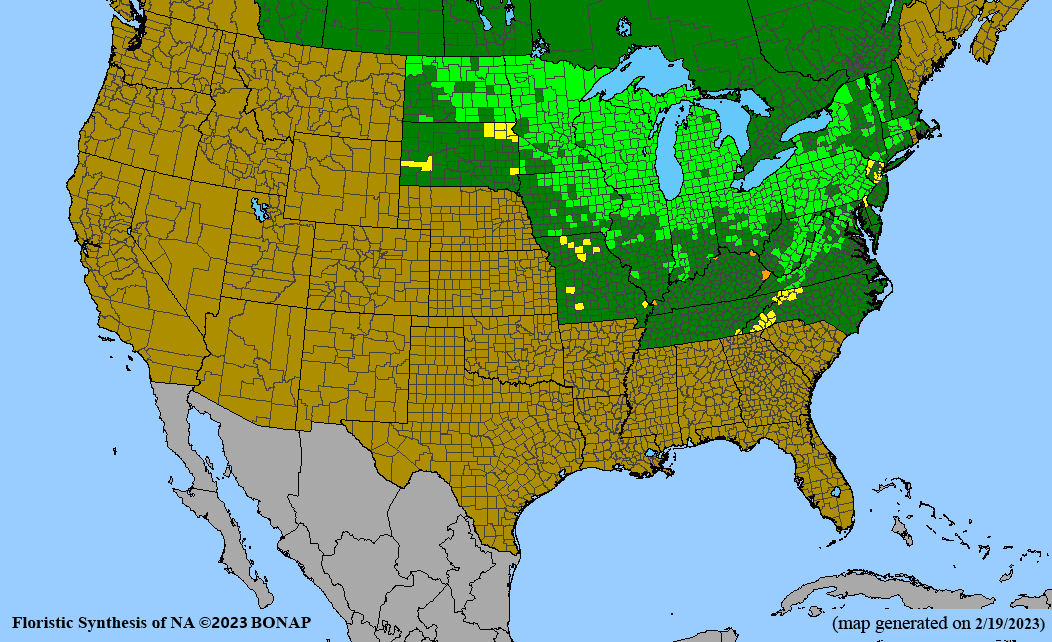
General Comments
The long flowering time and abundance of nectar and pollen make this an important food plant for many kinds of bees as well as small butterflies, wasps, beetles, and flies. We have observed the endangered Rusty Patched Bumble Bee visiting the flowers in white meadowsweet seed production plots. The dense colonies of stems provide shelter and nesting habitat for some bird species. The leaves, stems, and/or roots have uses in the traditional medicine and foodways of several Indigenous groups within the plant’s native range. Recommended for use as a low hedge, in perennial borders, wet prairie restorations, and roadside plantings.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: 45 days cold-moist stratification.
Sowing: Seeds are small and must be surface-sown; stratified seed germinates quickly (starting 5 days from sowing).
Transplanting: Seedling plugs (2.5 in deep, 73-cell trays) are ready to transplant about 12 weeks from germination. After several weeks in plugs, seedlings benefit from fertilizer application such as a sprinkling of coated fertilizer pellets. Harden off outside, then dibble into a weed barrier in irrigated production rows.
- Stand management
Weeds: Few issues if weed barrier used in planting year; dense foliage shades out most weeds in subsequent years; mow and trim between rows.
Pests: A few stems are affected by dark colored aphids that cause distortion of leaves and growing shoot tips.
Diseases: None noted.
Note: Mow plots down to 4 in during the dormant season every other year to stimulate production of robust new stems.
- Seed production
 First harvest: second year
First harvest: second yearYield: 25-90 pounds/acre (based on 2 plots)
Stand life: at least 8 years
Flowering date: June - August
Seed maturity/Harvest date: late October - early November
Seed retention: Shattering of seed from open capsules begins in late October to early November.
Harvest date range at TPC (2017-2023): Oct 17 - Nov 1
Recommended harvest method: Check plots frequently from mid-October through early November; hand clip or combine when follicles (dry fruits) have split open on most stalks.
- Seed cleaning and storage
Cleaning process: Do NOT use a brush machine. Brushing pulverizes the dried leaves, making it very difficult to extract the fine seed. Hand-clipped material can be beaten in a cloth bag to release seed. Combined or hand collected material can then be treated in the same way: run through 1/4 in hardware cloth to remove sticks, then airscreen. If greater purity is desired, passing the cleaned seed through soil sieves can remove residual chopped leaf material.
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zones 1 and 2
- References
Chayka, K. (n.d.). Spiraea alba (white meadowsweet). Minnesota Wildflowers. https://www.minnesotawildflowers.info/flower/white-meadowsweet
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). White meadowsweet. In Prairie plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 314). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Meadowsweet - Spiraea alba. Illinois Wildflowers. https://www.illinoiswildflowers.info/wetland/plants/meadowsweet.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
Michigan State University Extension (n.d.) Meadowsweet. MSU Extension Native Plants and Ecosystem Services. https://www.canr.msu.edu/nativeplants/plant_facts/meadowsweet
Runkel, S. T., & Roosa, D. M. (2009). Meadow sweet. In Wildflowers of the tallgrass prairie: The upper Midwest (2nd ed., pp. 142–143). University of Iowa Press.
USDA NRCS National Plant Data Team. (n.d.). Spiraea alba Du Roi. USDA plants database. https://plants.usda.gov/plant-profile/SPAL2
Species Guide Updated 12/18/2024
Sedges and Rushes
Sedges and Rushes sagemThe Species Production Guides for sedges and rushes provide specific information about growing each of these species for seed production, based primarily on direct experience in TPC production fields.
Scroll the list (alphabetized by scientific name) or press ctrl-f (or command-f) to search for any name in this page.
A printable file (pdf) is also provided on each completed species page for those needing a print version.
This section is a work in progress. We will continue to add new species guides as they are completed. At this time, two upland and three wetland Carex species are complete. Propagation of other species in these categories may be similar. The methods suggested for Carex bicknellii and C. molesta, for example, may be applied to other tufted, upland species such as C. brevior.
Carex annectens / yellowfruit sedge
Carex bebbii / Bebb's sedge
Carex brevior / shortbeak sedge
Carex cristatella / crested sedge
Carex gravida / heavy sedge
Carex interior / inland sedge
Carex laeviconica / smoothcone sedge
Carex pellita / woolly sedge
Carex sartwellii / Sartwell's sedge
Carex stricta / upright sedge
Carex suberecta / prairie straw sedge
Carex tribuloides / blunt broom sedge
Carex vulpinoidea / fox sedge
Scirpus atrovirens / green bulrush
Bicknell's sedge
Bicknell's sedge dickeye
Carex bicknellii Britton
Alternate Common Names: Bicknell’s oval sedge, copper-shouldered oval sedge
Scientific Synonyms: Carex bicknellii Britton var. bicknellii, Carex brevior. (Dewey) Mack var. crawei (W. Boott) B. Boivin, Carex straminea var. crawei Boott, Carex straminea Willd. var. meadii Boott
Family: sedge family (Cyperaceae)
Functional Group: sedges and rushes
Description
- Life cycle and growth form
perennial, short black rhizomes, forms bunches
Height: 1-4 ft

- Leaves and stem
3-4 leaves per stem, alternate, three-ranked, rough margins, pale green, flat and thin
- Flower, fruit and seedhead
Flower/fruit/seedhead: Erect to arching seed heads 2-6 cm long; 3-6 oval spikes with cone-shaped bases, each 10-18 mm long, per stem.
Pollination: wind
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 17,000 seeds/oz (IA NRCS)
1000 seed weight: 1.31g (Seed Information Database)
Description: Achene broadly elliptical, brown; mature perigynium (sac-like structure around the achene) flattened, with a translucent and membranous wing, distinct, parallel veins, and coppery-brown “shoulders,” the source of one alternate common name, “copper-shouldered oval sedge.”
Typical seed test
PLS: 84% (n = 11)
Purity: 98% (n = 10)
Germination: 30% (n = 7)
Dormancy: 61% (n = 7)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to moist soil; full sun; prairies, rock outcrops, savannas, along railroads; Wetland Indicator Status is Facultative Upland (FACU) for the Midwest.
Conservation status: Global- G5, secure; Delaware- SX, presumed extirpated; Vermont- SH, possibly extirpated; Arkansas, Maine, New Hampshire, Pennsylvania, and South Carolina- S1, critically imperiled; Massachusetts- S1/S2, critically imperiled to imperiled; New Jersey and Ohio- S2, imperiled (NatureServe)
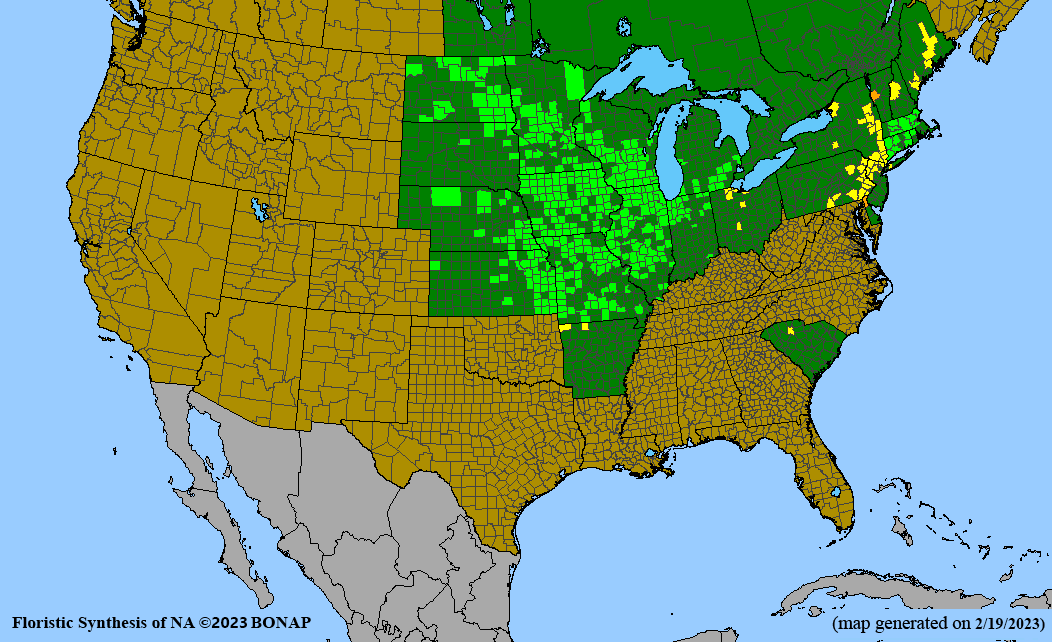
General Comments
Sedges are a large, diverse group of grass-like plants that are important components of prairies, wetlands, and woodlands across our region. In Iowa alone, there are about 120 species of sedges. Grasshoppers and the larvae of skipper butterflies, among other insects, feed on sedge foliage, and their seeds are eaten by grassland birds. They are notoriously difficult to identify to species, especially the oval sedges to which Bicknell’s sedge belongs. The development of stock seed by the Tallgrass Prairie Center in the early 2000s enabled broader access to reliably identified sedge species by native seed growers. The large, winged perigynia of Carex bicknellii, with their pearly color and translucence, make this species somewhat easier to identify than other oval sedges. This species is also one of a few oval sedges that are commonly found in upland prairie habitats. Seed production plots of Carex bicknellii in mesic to dry mesic soils do not require irrigation.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: 30 days cold-moist stratification; removal of perigynia produces a similar effect as stratification on germination in this species.
Sowing: Sow in germination flats or directly into plugs (2-3 seeds per cell), covering seed lightly (light improves germination of many sedge species); maintain even moisture until germination. Daytime temperatures should be around 70-80°F (22-27°C) and allowed to drop at night to 50-60°F (10-15°C). We have had good success planting into 2.5 in deep, 73-cell plug flats that are ridged to direct root development downward and have 3/4 in bottom openings to encourage root pruning and the formation of firmly rooted plugs for transplanting.
Transplanting: Seedlings are ready to transplant to the field about 10 weeks after sowing. Pop out a few plugs to check for adequate root development that will provide sturdy plugs for planting. A week or two before transplanting, move flats outside to ‘harden off.’
- Stand management
Weeds: Plastic mulch reduces weed pressure in the first year or more. Holes in the plastic should be widened somewhat in subsequent years to allow the bunches to expand. The most significant weed issue can be the presence of other oval sedges such as Carex brevior and Carex molesta, since they are competitive and their seed is difficult to distinguish from Carex bicknellii in the field and practically impossible to clean out of harvested seed. Obtaining clean, reliably identified, certified stock seed helps to prevent this issue.
Pests: None noted.
Diseases: None noted.
- Seed production
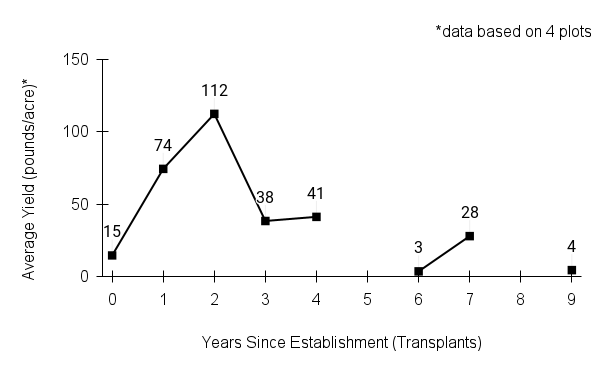
First harvest: There may be a small amount of seed in the first season, but most plants begin flowering and producing seed in their second growing season after transplanting.
Yield: Peak harvests are in the second through fourth years after transplanting, with yields from 40-112 pounds per acre, extrapolated from harvests of four plots grown at the Tallgrass Prairie Center.
Stand life: Plants may persist for up to ten years or more, but productive stand life is about five years, after which our yields have declined.
Flowering date: June in northeast Iowa
Seed maturity: Mid-late June to early July
Seed retention: Significant shattering occurs in high winds when perigynia are mature; lodging can also occur due to heavy rains/storms, complicating combine harvest.
Harvest date range at TPC (2007-2023): June 14 - July 31
Recommended harvest method: Combine when mature; a good rule of thumb is to wait until about 10% of seed heads have begun shattering.
- Seed cleaning and storage
Cleaning process: Air-dry seed for two weeks or more after harvest. Pass material through a coarse screen (1/2 in hardware cloth) to remove larger stemmy material, if needed, then air screen. If perigynia removal is desired, pass material through a brush machine with medium bristles before air screening. (Note: perigynia removal destroys several characteristics used in identification.)
Seed storage: cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern IA) and Zone 2 (central IA)
- References
Boeck Crew, C.M., Myers, M.C., Sherrard, M.E., Elgersma, K.J., Houseal, G.A., & Smith D.D. (2020). Stratification and perigynia removal improve total germination and germination speed in 3 upland prairie sedge species. Native Plants Journal, 21(2), 120-131. https://doi.org/10.3368/npj.21.2.120
Chayka, Katy. (n.d.). Carex bicknellii (Bicknell’s sedge). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/bicknells-sedge
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Bicknell’s Sedge. In Prairie Plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 160). University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Bicknell’s sedge - Carex bicknellii. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/bicknell_sedge.htm
Houseal, G. (2010). Plasticulture for seed production of wetland (Carex) species. Native Plants Journal, 11(1), 58-64. https://doi.org/10.2979/NPJ.2010.11.1.58
Mastrogiuseppe, J., Rothrock, P. E., Dibble, A. C., & Reznicek, A. A. (2020, November 5). Carex bicknellii Britton. Flora of North America. http://floranorthamerica.org/Carex_bicknellii
Mohlenbrock, R. H. (1999). Carex bicknellii. In Illustrated Flora of Illinois - Sedges: Carex (p. 139). Southern Illinois University Press.
Murphy, M. & Spyreas, G. & Marcum, P. (2025) Carex of Illinois & Surrounding States: The Oval Sedges. University of Illinois Press
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 28, 2024).
Schütz, W. & Rave, G. (1999). The effect of cold stratification and light on the seed germination of temperate sedges (Carex) from various habitats and implications for regenerative strategies. Plant Ecology 144, 215–230. https://doi.org/10.1023/A:1009892004730
USDA NRCS National Plant Data Team. (n.d.). Carex bicknellii Britton. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=CABI3
Species Guide Updated 2/5/2025
Buxbaum's sedge
Buxbaum's sedge dickeye
Carex buxbaumii, Wahlenb.
Alternate Common Name: brown bog sedge
Scientific Synonym(s): Carex buxbaumii var. anticostensis Raymond, Carex holmiana, Carex polygama Mackenzie
Family: sedge family (Cyperaceae)
Functional Group: sedges and rushes
Description
- Life cycle and growth form
Perennial, long-rhizomatous, forming large stands with a distinctive bluish-green color compared with the yellow-green of most sedges.
Height: 1-2.5 ft
- Leaves and stem
Leaves flat, hairless, and rough on the edges; lower sheaths are rusty brown and ladder fibrillose (made of evenly spaced, linked fibers) near their tops, flowering stems are stiff and 3-sided, smooth.
- Flower, fruit and seedhead
Fruit/seedhead: 1-4 spikes per plant; pistillate spikes bear up to 40 perigynia per spike, distinctive scales are red-purple to dark brown with a green midvein, perigynia (papery coverings around each “seed”) are hairless and gray-green, can be beakless or have a tiny beak, “seeds” (achenes) are 3-sided.
Pollination: wind

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 14,500 (IA NRCS)
1000 seed weight: 1.66 g (Seed Information Database)
Description: Seed unit is an achene (2 mm long) with surrounding perigynium (1.5 x 3 mm); perigynia are gray-green with lighter veins and a very short beak; achene is three-sided and brown.
Typical seed test
unknown at this time.
- Habitat and range
Habitat: Mesic to wet soil; partial to full sun; prairie swales, meadows, fens, marshes, ditches; Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest; irrigation is recommended for seed production.
Conservation status: Global- G5, secure; District of Columbia and Georgia- SH, possibly extirpated; Arkansas, Connecticut, Delaware, Kansas, Kentucky, New Hampshire, South Carolina, South Dakota, Tennessee, and Vermont- S1, critically imperiled; Maryland, Missouri, Nebraska, Nevada, Massachusetts, New York, North Carolina, North Dakota, Virginia, and West Virginia- S2, imperiled; California, Idaho, New Jersey, Pennsylvania, Washington, Wyoming, and Illinois- S3, vulnerable (NatureServe)
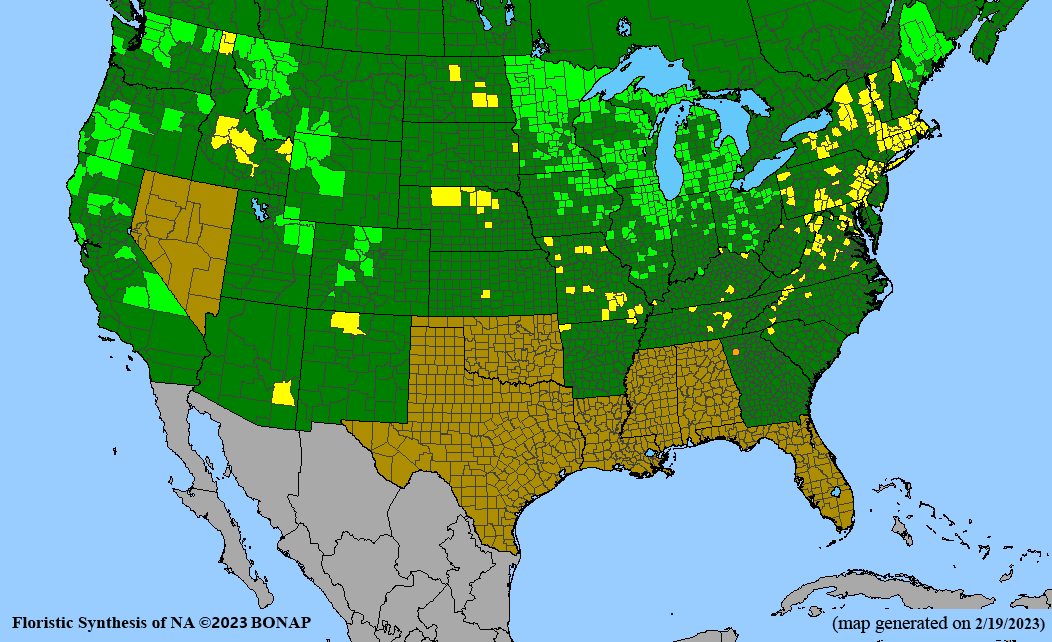
General Comments
Sedges are a large, diverse group of grass-like plants that are important components of prairies, wetlands, and woodlands across our region. In Iowa alone, there are about 120 species of sedges. Grasshoppers and the larvae of skippers and other butterflies and moths feed on wetland sedge foliage, and their seeds are eaten by waterfowl and other birds. Sedges are notoriously difficult to identify to species. Buxbaum’s sedge is distinguishable by, among other characteristics, its rhizomatous habit, bluish green foliage, and dark purplish-brown scales in the seedheads (spikelets). The development of stock seed by the Tallgrass Prairie Center in the early 2000s enabled broader access to reliably identified sedge species by native seed growers. As an obligate wetland species, Buxbaum’s sedge benefits from irrigation in production settings.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Benefits from cold-moist stratification for at least 60 days.
Sowing: Sow in germination flats, covering seed lightly (light improves germination of many sedge species); germination may be slow and sporadic. Daytime temperatures should be around 70-80°F (22-27°C) and allowed to drop at night to 50-60°F (10-15°C). We have had good success dibbling seedlings into 2.5 in deep, 73-cell plug flats that are ridged to direct root development downward and have 3/4 in bottom openings to encourage root pruning and the formation of firmly rooted plugs for transplanting.
Transplanting: Seedlings are ready to transplant to the field about 14-16 weeks after sowing. Pop out a few plugs to check for adequate root development that will provide sturdy plugs for planting. A week or two before transplanting, move flats outside to ‘harden off.’
- Stand management
Weeds: Plastic mulch reduces weed pressure in the first year or more. Plastic must eventually be removed to allow the plants to spread by rhizomes. A well-established stand outcompetes many weeds. In large-scale production systems or those where the use of weed barriers and/or hand weeding is not practical, broad-leaf herbicides and/or pre-emergent herbicides may be useful to prevent weeds from competing with the sedge plants and/or complicating the seed cleaning process. Significant weed problems may be caused by winter annuals (e.g., members of the mustard family), other small-seeded broad-leaf annuals, and annual grasses (e.g., downy brome). Herbicide applications should be timed to most effectively control specific weeds and minimize damage to the sedge plants. Care must be taken to read affected “weed” lists, as sedges are considered weeds in crop systems. Always read and follow label instructions.
Pests: None noted.
Diseases: None noted.
- Seed production
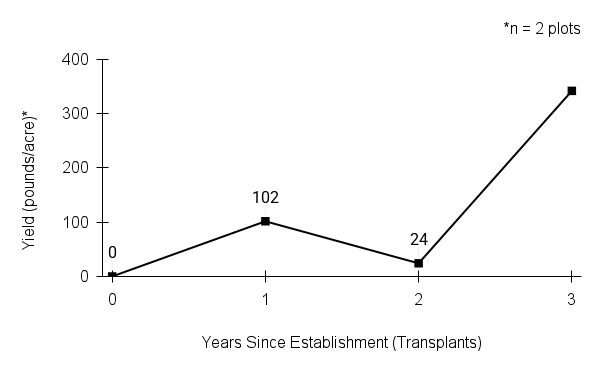 First harvest: Small amounts may be produced in the first and second years after transplanting.
First harvest: Small amounts may be produced in the first and second years after transplanting.Yield/acre: 24-340 lbs per acre
Stand life: Appears to take 3 years to reach peak production; plants are likely long-lived, but uncertain whether yields will be sustained or sporadic; mowing in fall or winter may improve yields in subsequent seasons.
Flowering date: May in northern Iowa
Seed maturity/harvest date: Mid to late June in NE Iowa.
Seed retention: Moderate shattering observed.
Harvest date range at TPC (2010-2011, 2025): June 16 - 27
Recommended harvest method: Combine at maturity; seed threshes cleanly.
- Seed cleaning and storage
Cleaning process: Air-dry combined material for two weeks or more after harvest. Pass material through a coarse screen (1/2 in hardware cloth) to remove larger stemmy material, if needed, then air screen.
Seed storage: Cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone Iowa
- References
Carex buxbaumii (Buxbaum’s Sedge). Minnesota Wildflowers. (n.d.). https://www.minnesotawildflowers.info/grass-sedge-rush/buxbaums-sedge
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Buxbaum’s Sedge. In Prairie Plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 161). essay, University of Wisconsin-Madison Arboretum.
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Murray, D. F. (2020, November 5). Carex buxbaumii Wahlenberg. Flora of North America. http://floranorthamerica.org/Carex_buxbaumii
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
USDA NRCS National Plant Data Team. (n.d.). Carex buxbaumii Wahlenb.. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=CABU6
Species Guide Updated 12/2/2024
Dudley's rush
Dudley's rush dickeye
Juncus dudleyi, Wiegand
Scientific Synonyms: Juncus tenuis Willd. var. dudleyi (Wiegand) F.J. Herm., Juncus tenuis Willd. var. uniflorus auct. non Farw. p.p.
Family: rush family (Juncaceae)
Functional Group: sedges and rushes
Description
- Life cycle and growth form
Perennial, bunch forming rush with fine fibrous roots and short rhizomes.
Height: 1-2 ft

- Leaves and stem

Leaf blades flat, dark green, up to 1 mm wide and 12 in long, 2-3 leaves per flowering stem; flowering stems longer than the blades, round in cross-section; small appendages where the leaf blade meets the sheath (auricles) are thickened, rounded, often yellowish, helping to distinguish Dudley’s rush from similar species that have auricles that are elongated and membranous (J. tenuis) or rounded and papery (J. interior).
- Flower, fruit and seedhead
Fruit/seed head: Inflorescence is a dense to loose cluster of up to 80 small green, 6-parted flowers that mature into oval brown capsules surrounded by 6 persistent, sharply pointed tepals; usually at least one slender, thread-like bract from the base of the inflorescence extends beyond the flowers; capsules open at maturity to release numerous, very tiny seeds.
Pollination: wind

- Seed
 Seed image includes a ruler with millimeter markings.
Seed image includes a ruler with millimeter markings.Seed characteristics
Seed weight:
Seeds per ounce: 3,200,000 (IA NRCS)
1000 seed weight: 0.01 g (Seed Information Database)
Description: Seeds are a slightly curved oval or crescent shape, translucent red-gold in color with a whitish bump on one end (but not a long “tail”).
Typical seed test
PLS: 84% (n=5)
Purity: 99.8% (n=5)
Germination: 1.2% (n=5)
Dormancy: 77.4% (n=5)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist soils, full sun; wet prairies, fens, marshes, seeps, wet ditches, shores; Wetland Status is FACW (usually occurring in wetlands) for the Midwest; irrigation is recommended for seed production.
Conservation status: Global- G5, secure; Illinois, New Jersey- S3, vulnerable; Alabama, North Carolina, Nevada- S1, critically imperiled; remaining states either S5, secure, S4, apparently secure, or unranked (NatureServe).
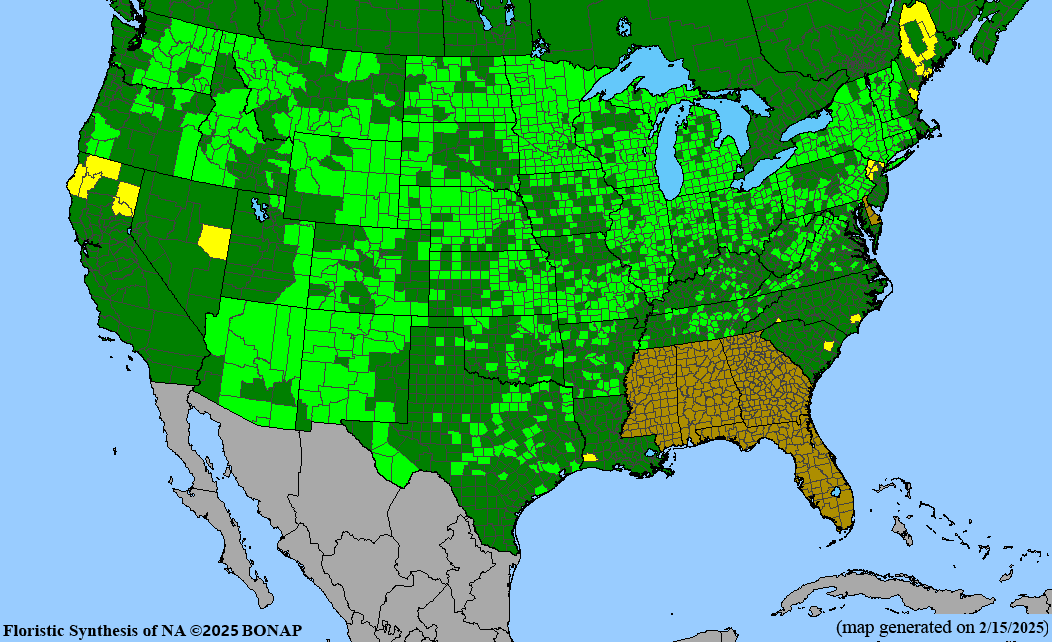
General Comments
Rushes are cool season, somewhat grass-like plants that are usually found in habitats with wet soils. When making collections in remnant habitats for development of Iowa Source Identified seed of Dudley’s rush, we found the plants in both disturbed and high quality prairies, fens, marshes, and ditches. The tiny seeds are sticky when wet and may be dispersed by sticking to the feet of waterfowl and mammals. In our region, there are three species that are very similar, and Dudley’s rush is distinguished primarily by its auricles - little flaps of tissue where the leaf sheath meets the blade - which are thickened and often yellowish in Dudley’s rush in comparison with path rush (J. tenuis) and inland rush (J. interior).
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production and do not recommend it.
Greenhouse
Seed pre-treatment: 60-day cold/moist stratification.
Sowing: Surface sow in greenhouse about 2-3 months before last frost. Seed is exceptionally tiny, so take care in watering (mist gently) to avoid dislodging. Germination is greatly improved by keeping flats saturated. Seedlings are tiny and threadlike but grow vigorously.
Transplanting: When plugs are well-rooted, move them outside to harden off, then transplant at 8-12 in intervals in plastic mulch with irrigation tape.
- Stand management
Weeds: Prepare a clean, weed-free bed. Plastic mulch suppresses weeds in the first year or two. Mow or cultivate between rows. Hand-hoe and rogue within rows. Contamination of seed by weedy species is unlikely due to the extremely small size of Dudley’s rush seed enabling removal of weeds in seed cleaning.
Pests: None noted.
Diseases: None noted.
- Seed production
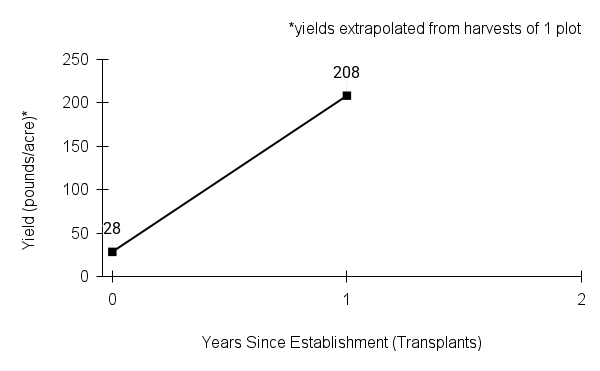 First Harvest: Some flowering and seed set may be expected at the end of the first season from transplants.
First Harvest: Some flowering and seed set may be expected at the end of the first season from transplants.Yield/Acre: 20-200 pounds per acre (*extrapolated from harvests of one plot)
Stand Life: Unknown at this time but likely 3-5 years. Plants are relatively small, and invasion of plots by larger, competitive weeds is likely to cause loss of yield over time.
Flowering Date: late May to early June in northeast Iowa
Seed Maturity/Harvest Date: early to mid July in northeast Iowa
Seed retention: Moderate risk of shattering; some seed is lost as soon as capsules open, but some is retained.
Harvest date range at TPC (2024-2025): July 3 - Sept 25 (flowering and seed set greatly delayed in establishment year)
Recommended Harvest Method: combine
- Seed cleaning and storage
Cleaning process: Brush to free any seed remaining in capsules, then airscreen. Use the smallest sifting screen available (or a solid screen), check for seed in the sifting fraction and rescreen. It can be difficult to separate dust from the seed; adjust air and screen multiple times if needed. Eliminating the brushing step would likely produce less dust and improve purity of the final product with slight loss of yield.
Seed storage: Cool/dry (33-50° F, 30-50% RH); seed longevity in storage unknown at this time.
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone NI (northern Iowa, in alignment with the Generalized Provisional Seed Zones of the US Forest Service)
- References
Bower, Andrew D.; St.Clair, J. Bradley; Erickson, Vicky. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Chayka, K. (n.d.). Juncus dudleyi (Dudley's Rush). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/dudleys-rush
Hilty, J. (2019). Dudley's Rush - Juncus dudleyi. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/dd_rush.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2025. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: December 18, 2025).
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. (2023) Seed Information Database (SID). Available from: https://ser-sid.org/ (February 2023)
USDA-NRCS. (2024). Native seeding calculator 2024 [Excel File]. Retrieved from https://efotg.sc.egov.usda.gov/#/state/IA/documents/section=4&folder=-6
USDA NRCS National Plant Data Team. (n.d.). Juncus dudleyi Wiegand, Dudley's rush. USDA plants database. https://plants.usda.gov/plant-profile/JUDU2
Species Guide Updated 12/18/2025
bottlebrush sedge
bottlebrush sedge dickeye
Carex hystericina Muhl. ex Willd.
Alternate Common Name: porcupine sedge
Scientific Synonym: Carex hystricina Muhl. Rydberg.
Family: sedge family (Cyperaceae)
Functional Group: sedges and rushes
Description
- Life cycle and growth form
Perennial with fibrous roots, forms clumps.
Height: 3/4 - 3 ft
- Leaves and stem
Leaves in alternate, 3-ranked arrangement, hairless; basal sheaths are reddish purple and fibrous; culms are stiff and 3-sided, hairless, unbranched.
- Flower, fruit and seedhead
Fruit/seedhead: Seed head consists of a terminal male spike and one to four prickly, cylindrical pistillate spikes, each containing 40-100 perigynia (seed containing structures).
Pollination: wind

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 30,000 seeds/oz (IA NRCS)
1000 seed weight: 0.48g (Seed Information Database)
Description: “Seeds” are 3-sided, ovate, brown achenes, wrapped in an inflated, distinctly veined perigynium with a long beak and two short teeth.
Typical seed test
PLS: 87.48% (n=1)
Purity: 99.41% (n=1)
Germination: 62% (n=1)
Dormancy: 26% (n=1, determined by TZ)
(based on one test of a seedlot grown at TPC)
- Habitat and range
Habitat: Moist to wet soil; partial to full sun; prairies, meadows, seeps, fens, marshes, swamps, ditches; Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest.
Conservation status: Global- G5, secure; District of Columbia and Kentucky- SH, possibly extirpated; Maryland- S1, critically imperiled; California and Georgia- S2, imperiled; Arizona, Kansas, Washington, West Virginia, Illinois, and Wyoming- S3, vulnerable (NatureServe)
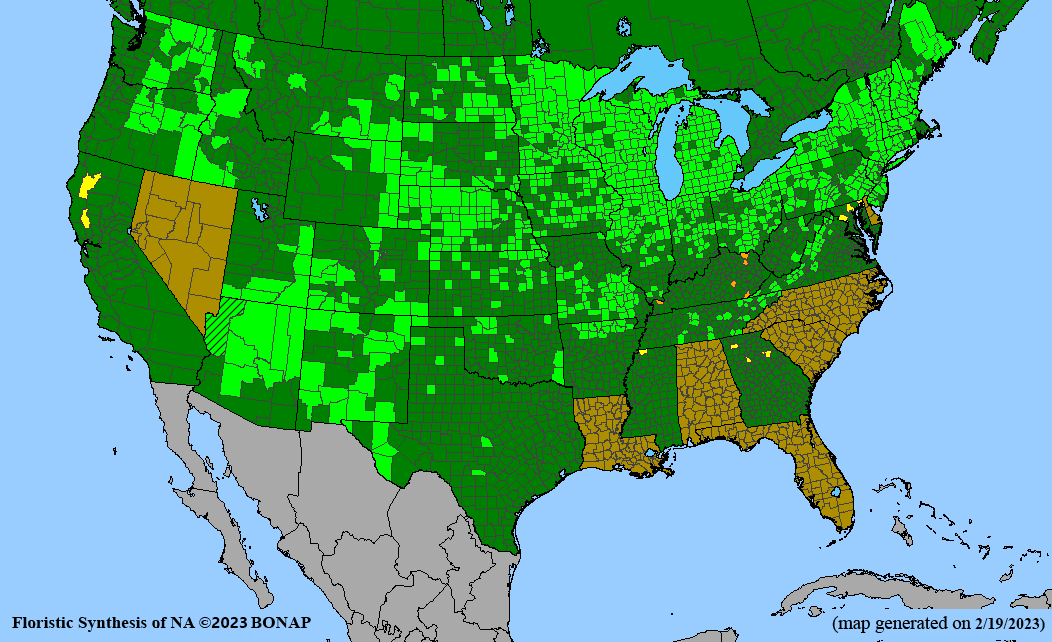
General Comments
Sedges are a large, diverse group of grass-like plants that are important components of prairies, wetlands, and woodlands across our region. In Iowa alone, there are about 120 species of sedges. Grasshoppers and the larvae of skippers and other butterflies and moths feed on wetland sedge foliage, and their seeds are eaten by waterfowl and other birds. Sedges are notoriously difficult to identify to species. The prickly, cylindrical spikes of bottlebrush sedge are somewhat distinctive, but this species can be confused with longhair or bristly sedge (Carex comosa) which also occurs in our region and sallow sedge (Carex lurida) which is a state listed species in Iowa but is more common in neighboring states to the south and east. The development of stock seed by the Tallgrass Prairie Center in the early 2000s enabled broader access to reliably identified sedge species by native seed growers. As an obligate wetland species, bottlebrush sedge benefits from irrigation in production settings.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: Benefits from cold-moist stratification for 30 days.
Sowing: Sow in germination flats or directly into plugs (2-3 seeds per cell), covering seed lightly (light improves germination of many sedge species); maintain even moisture until germination. Daytime temperatures should be around 70-80°F (22-27°C) and allowed to drop at night to 50-60°F (10-15°C). We have had good success planting into 2.5 in deep, 73-cell plug flats that are ridged to direct root development downward and have 3/4 in bottom openings to encourage root pruning and the formation of firmly rooted plugs for transplanting.
Transplanting: Seedlings are ready to transplant to the field about 10 weeks after sowing. Pop out a few plugs to check for adequate root development that will provide sturdy plugs for planting. A week or two before transplanting, move flats outside to ‘harden off.’
- Stand management
Weeds: Plastic mulch reduces weed pressure in the first year or more. Holes in the plastic should be widened somewhat in subsequent years to allow the bunches to expand. Bunching plants are robust and leafy, competing well with many weeds; we have interplanted porcupine sedge with wetland forbs to provide support and reduce weed pressure.
Pests: None noted.
Diseases: None noted.
Soil moisture: Irrigation is recommended, though bottlebrush sedge may require less supplemental water than other wetland sedges. Drip tape can be applied under plastic mulch as planting beds are formed.
- Seed production
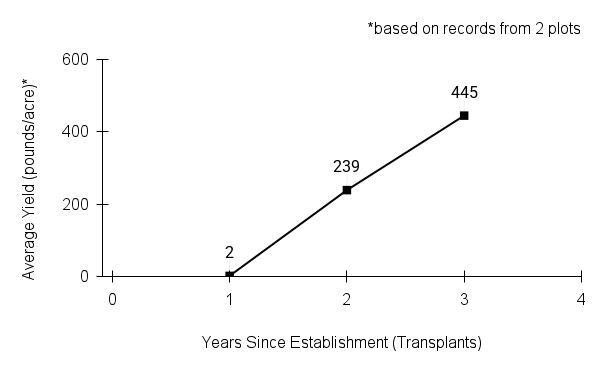
First harvest: Plants produce a small amount of seed in the year following the transplant year. Peak harvest may be in the third year after transplanting.
Yield: At peak (3 years after planting), yields are around 445 lbs per acre (extrapolated from two production plots).
Stand life: Plants may be long-lived, but long-term yields are unknown.
Flowering date: June-July
Seed maturity/harvest date: Early to mid-July in northeast Iowa.
Seed retention: Seed begins to shatter in mid-July.
Harvest date range at TPC (2012-2024): June 26 - July 22
Recommended harvest method: Combine larger plantings. Hand harvest is effective for small plantings, and seed threshes easily from stems once dried.
- Seed cleaning and storage
Cleaning process: Air-dry seed for two weeks or more after harvest. Pass material through a coarse screen (1/2 in hardware cloth) to remove larger stemmy material, if needed, then air screen.
Seed storage: Cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone IA
- References
Carex hystericina (Porcupine Sedge). Minnesota Wildflowers. (n.d.). https://www.minnesotawildflowers.info/grass-sedge-rush/porcupine-sedge
Eggers, S. D., & Reed, D. M. (1997). Shallow Marshes. In Wetland Plants and Plant Communities of Minnesota and Wisconsin (2nd ed., p. 82). essay, U.S. Army Corps of Engineers, St. Paul District
Great Plains Flora Association. (1991). Sedge Family. In Flora of the Great Plains (2nd ed., p. 1080). University Press of Kansas.
Houseal, G. (2010). Plasticulture for seed production of wetland (Carex) species. Native Plants Journal, 11(1), 58-64. https://doi.org/10.2979/NPJ.2010.11.1.58
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Kettenring KM, Gardner G, Galatowitsch SM. Effect of light on seed germination of eight wetland Carex species. Ann Bot. 2006 Oct;98(4):869-74. doi: 10.1093/aob/mcl170. Epub 2006 Aug 11. PMID: 16905568; PMCID: PMC2806167.
Kettering, K.M. & Galatowitsch S.M. (2007) Temperature requirements for dormancy break and seed germination vary greatly among 14 wetland Carex species. Aquatic Botany, 87(3), 209 -220. https://doi.org/10.1016/j.aquabot.2007.06.003
Mohlenbrock, R. H. (1999). Carex hystericina. In Illustrated Flora of Illinois - Sedges: Carex (p. 285). Southern Illinois University Press.
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024)..
Porcupine Sedge. Illinois Wildflowers. (n.d.). https://www.illinoiswildflowers.info/grasses/plants/porc_sedge.htm
USDA NRCS National Plant Data Team. (n.d.). Carex hystericina Muhl. ex Willd.. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=CAHY4
Species Guide Updated 12/1/2025
broom sedge
broom sedge dickeye
Carex scoparia Schkuhr ex Willd.
Alternate Common Names: pointed broom sedge, lance-fruited oval sedge
Scientific Synonyms: Carex scoparia Schkuhr var. moniliformis Tuckerm., Carex scoparia Schkuhr var. condensa Fern.
Family: sedge family (Cyperaceae)
Functional Group: sedges and rushes
Description
- Life cycle and growth form
perennial, fibrous rooted, short-rhizomatous, clump forming
Height: 1-2.5 ft
- Leaves and stem
Leaves flat and hairless with rough margins, alternate arrangement, 3-ranked, shorter than flowering stems; top of sheath around flowering stem (culm) has a ‘u’ or ‘v’ shaped notch, basal sheaths are brown and fibrous; culm is hairless and 3-sided, smooth and unbranched.
- Flower, fruit and seedhead
Fruit/seedhead: 3-10 spikes per culm, each up to 2/3 in (10-15 mm) long, often crowded at the end of the culm but still distinct as individual elliptical to oval spikes; golden-tan color at maturity, stem may bend or arch at the tip or be straight.
Pollination: wind
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 84,000 seeds/oz (IA NRCS)
1000 seed weight: 0.44g (Seed Information Database)
Description: “seed” is an ovate to elliptic, brown achene that is enclosed in a perigynium; perigynium is golden-tan, flat, and hairless, 5-veined on each side, lance-shaped and often 3-4 times as long as wide.
Typical seed test
PLS: 93% (n = 5)
Purity: 96% (n = 5)
Germination: 50% (n = 3)
Dormancy: 66% (n = 5)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soil; partial to full sun; prairies, shorelines, swales, fens, seeps, marshes, ditches; Wetland Indicator Status is Facultative Wetland (FACW) for the Midwest.
Conservation status: Global- G5, secure; North Dakota- SH, possibly extirpated; Arkansas, Utah, and Wyoming- S1, critically imperiled; Montana- S1/S2, critically imperiled to imperiled; Georgia- S3, vulnerable (NatureServe)
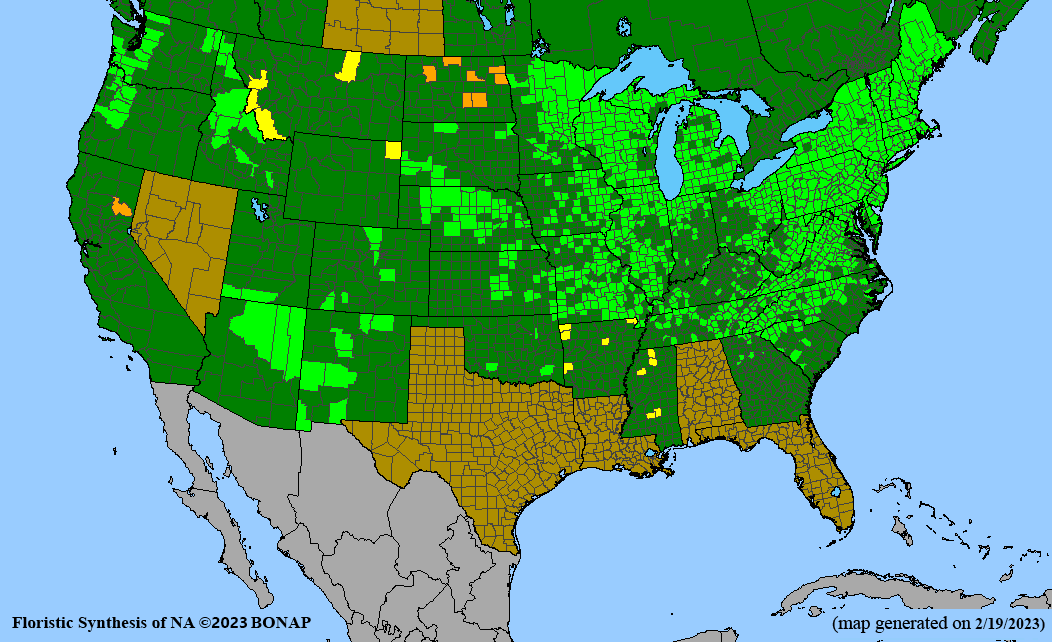
General Comments
Sedges are a large, diverse group of grass-like plants that are important components of prairies, wetlands, and woodlands across our region. In Iowa alone, there are about 120 species of sedges. Grasshoppers and the larvae of skippers and other butterflies and moths feed on sedge foliage, and their seeds are eaten by grassland birds and waterfowl. They are notoriously difficult to identify to species, especially the oval sedges to which broom sedge belongs. The development of stock seed by the Tallgrass Prairie Center in the early 2000s enabled broader access to reliably identified sedge species by native seed growers. Broom sedge is found naturally in moist to wet soils and may benefit from supplemental watering in seed production systems.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not recommend direct seeding for this species.
Greenhouse
Seed pre-treatment: Benefits from cold-moist stratification for 30 days.
Sowing: Sow in germination flats or directly into plugs (2-3 seeds per cell), covering seed lightly (light improves germination of many sedge species); maintain even moisture until germination. Daytime temperatures should be around 70-80°F (22-27°C) and allowed to drop at night to 50-60°F (10-15°C). We have had good success planting into 2.5 in deep, 73-cell plug flats that are ridged to direct root development downward and have 3/4 in bottom openings to encourage root pruning and the formation of firmly rooted plugs for transplanting.
Transplanting: Seedlings are ready to transplant to an irrigated field about 10 weeks after sowing. Pop out a few plugs to check for adequate root development that will provide sturdy plugs for planting. A week or two before transplanting, move flats outside to ‘harden off.’
- Stand management
Weeds: Plastic mulch reduces weed pressure in the first year or more. Holes in the plastic should be widened somewhat in subsequent years to allow the bunches to expand. Bunching plants are robust and leafy, competing well with many weeds; we have interplanted broom sedge with cardinalflower to provide support and reduce weed pressure (note: interplanting necessitates hand harvest of the sedge and eliminates broadleaf herbicides as an option). In large-scale production systems or those where the use of weed barriers and/or hand weeding is not practical, herbicides (e.g., broad-leaf herbicides and/or pre-emergents) may be useful to prevent weeds from competing with the sedge plants and/or complicating the seed cleaning process. Significant weed problems may be caused by winter annuals (e.g., members of the mustard family), other small-seeded broad-leaf annuals, and annual grasses (e.g., downy brome). Herbicide applications should be timed to most effectively control specific weeds and minimize damage to the sedge plants. Care must be taken to read affected “weed” lists, as sedges are considered weeds in crop systems. Always read and follow label instructions.
Pests: None noted.
Diseases: None noted.
Soil moisture: Irrigation is recommended. Drip tape can be applied under plastic mulch as planting beds are formed.
- Seed production
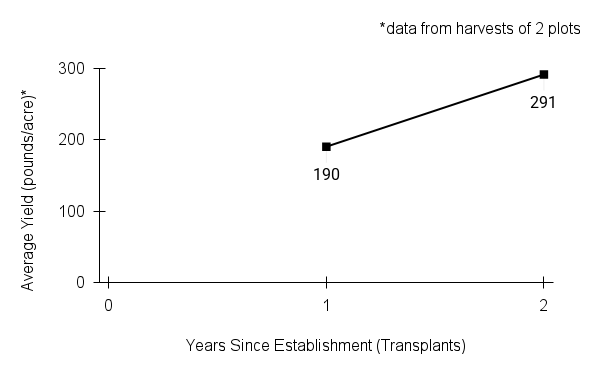 First harvest: First harvest one year after transplanting
First harvest: First harvest one year after transplantingYield/acre: 190-290 pounds per acre (extrapolated from cleaned seed harvested from two plots at TPC)
Stand life: Unknown, but plants are likely long-lived and should continue to be productive with proper weed management.
Flowering date: June
Seed maturity/Harvest date: Late June to early July in northeastern Iowa
Seed retention: Moderate risk of shattering at maturity; also vulnerable to lodging, potentially complicating combine harvest.
Harvest date range at TPC (2010-2023): June 28 - July 3
Recommended harvest method: We have harvested small plots using hand sickles; seed threshes easily after drying on tarps. Combining should also work well, if seed is mature.
- Seed cleaning and storage
Cleaning process: Air-dry seed for two weeks or more after harvest. Pass material through a coarse screen (1/2 in hardware cloth) to remove larger stemmy material, if needed, then air screen.
Seed storage: Cool/dry (33-50° F, 30-50% RH)
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone IA
- References
Carex scoparia (Pointed Broom Sedge). Minnesota Wildflowers. (n.d.). https://www.minnesotawildflowers.info/grass-sedge-rush/pointed-broom-sedge
Houseal, G. (2010). Plasticulture for seed production of wetland (Carex) species. Native Plants Journal, 11(1), 58-64. https://doi.org/10.2979/NPJ.2010.11.1.58
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Kettenring and Galatowitsch (2007) https://www.sciencedirect.com/science/article/pii/S030437700700085X
Kettenring KM, Gardner G, Galatowitsch SM. Effect of light on seed germination of eight wetland Carex species. Ann Bot. 2006 Oct;98(4):869-74. doi: 10.1093/aob/mcl170. Epub 2006 Aug 11. PMID: 16905568; PMCID: PMC2806167.
Mohlenbrock, R. H. (1999). Carex scoparia. In Illustrated Flora of Illinois - Sedges: Carex (p. 108). Southern Illinois University Press.
Murphy, M. & Spyreas, G. & Marcum, P. (2025) Carex of Illinois & Surrounding States: The Oval Sedges. University of Illinois Press
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Pointed Broom Sedge. Illinois Wildflowers. (n.d.). https://www.illinoiswildflowers.info/grasses/plants/pb_sedge.htm
USDA NRCS National Plant Data Team. (n.d.). Carex scoparia Schkuhr ex Willd.. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=CASC11
Species Guide Updated 2/7/2025
troublesome sedge
troublesome sedge dickeye
Carex molesta, Mack. ex Bright
Alternate Common Names: field oval sedge, pest sedge
Family: sedge family (Cyperaceae)
Functional Group: sedges and rushes
Description
- Life cycle and growth form
Perennial, clump-forming (cespitose) sedge, with fibrous roots.
Height: 1-3 ft
- Leaves and stem
Leaves flat and hairless with rough margins, 1.5-4 (less than 1/4 in) mm wide, shorter than flowering stalks; top of sheath is ‘u’ or ‘v’ shaped and white, base of sheath is brown and somewhat fibrous; flowering culms are hairless and 3-sided.
- Flower, fruit and seedhead
Flower: Tiny greenish flowers are clustered into 2-5 nearly globular spikes, each about 1/3 in long, at the tip of a culm.
Fruit/seed head: Spikes mature to clusters of achenes, each wrapped in a perigynium, separated by delicate pistillate scales.
Pollination: Wind
Note: There are several excellent guides to the sedges of the Upper Midwest region, and it is worth having a selection of them on your bookshelf to use as references, as questions about the identity of sedges often arise, even in carefully planted production fields. This description is intended as an introduction, not a complete, definitive guide.
- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 25,000 (IA NRCS)
1000 seed weight: 1.11g (Seed Information Database)
Description: “Seed” is a two-sided achene, brown, elliptic, 0.9-1.3 mm wide and 1.3-1.7 mm long, enclosed in a flattened, ovate, brown to greenish perigynium, 1.8-3 mm wide and 3.3-5 mm long, with a beak (0.7-1.6 mm long) and pale, flattened wing.
Typical seed test
PLS: 93% (n = 11)
Purity: 98% (n = 11)
Germination: 29% (n = 9)
Dormancy: 57% (n = 9)
(averages obtained from n tests of purchased seed lots)
- Habitat and range
Habitat: Dry to wet soil; partial to full sun; prairies, meadows, swamps, thickets, woodland edges and floodplains, river banks, ditches; Wetland Indicator Status is Facultative (FAC) for the Midwest; mesic to wet-mesic soils are recommended for seed production.
Conservation status: Global- G5, secure; New Hampshire- SH, possibly extirpated; Connecticut, Illinois, Massachusetts, and Vermont- S1, critically imperiled; New Jersey and West Virginia- S2/S3, imperiled to vulnerable; Kansas- S3, vulnerable; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
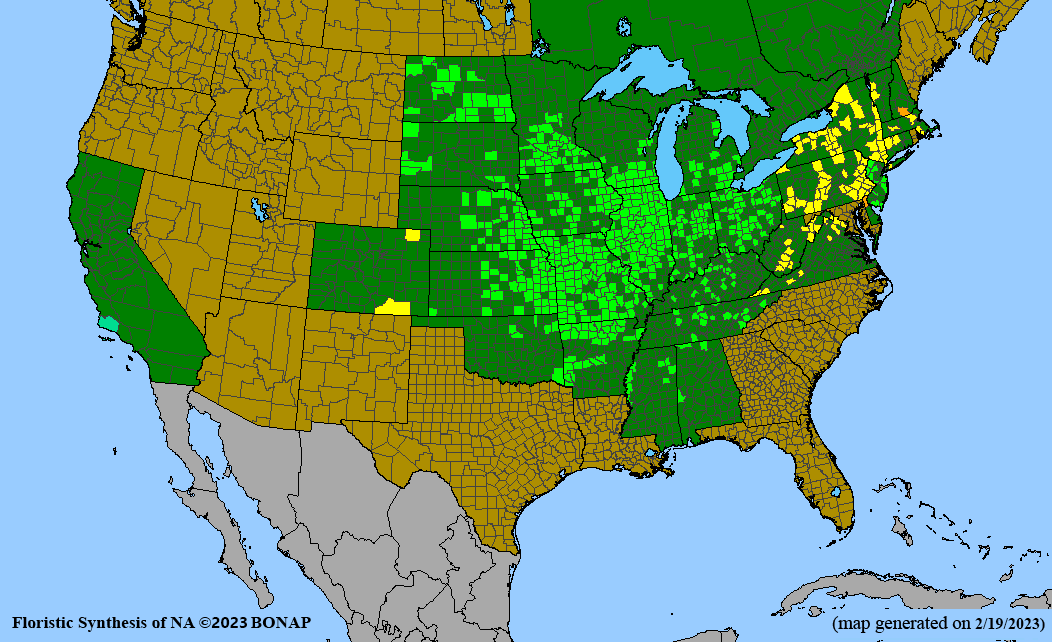
General Comments
Sedges are a large, diverse group of grass-like plants that are important components of prairies, wetlands, and woodlands across our region. In Iowa alone, there are about 120 species of sedges. Grasshoppers and the larvae of skipper butterflies, among other insects, feed on sedge foliage, and their seeds are eaten by grassland birds. Troublesome sedge is easy to propagate for seed production and reliably establishes from seed in prairie plantings. This species has a wide tolerance for soil moisture variation and persists in areas with some disturbance. Sedges are notoriously difficult to identify to species, especially the oval sedges, the section to which troublesome sedge belongs. The development of stock seed by the Tallgrass Prairie Center in the early 2000s enabled broader access to reliably identified sedge species by native seed growers.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
We do not have experience with direct seeding this species for seed production.
Greenhouse
Seed pre-treatment: 30 days cold-moist stratification
Sowing: Sow in germination flats or directly into plugs (2-3 seeds per cell), covering seed lightly (light improves germination of many sedge species); maintain even moisture until germination. Daytime temperatures should be around 70-80°F (22-27°C) and allowed to drop at night to 50-60°F (10-15°C). We have had good success planting into 2.5 in deep, 73-cell plug flats that are ridged to direct root development downward and have 3/4 in bottom openings to encourage root pruning and the formation of firmly rooted plugs for transplanting.
Transplanting: Seedlings are ready to transplant to the field about 10 weeks after sowing. Pop out a few plugs to check for adequate root development that will provide sturdy plugs for planting. A week or two before transplanting, move flats outside to ‘harden off.’ Transplant into prepared plasticulture rows at 8-12 inch spacing.
- Stand management
Weeds: Plastic mulch reduces weed pressure in the first year or more. Holes in the plastic should be widened somewhat in subsequent years to allow the bunches to expand. In large-scale production systems or those where the use of weed barriers and/or hand weeding is not practical, herbicides (e.g., broad-leaf herbicides and/or pre-emergents) may be useful to prevent weeds from competing with the sedge plants and/or complicating the seed cleaning process. Significant weed problems may be caused by winter annuals (e.g., members of the mustard family), other small-seeded broad-leaf annuals, and annual grasses (e.g., downy brome). Herbicide applications should be timed to most effectively control specific weeds and minimize damage to the sedge plants. Care must be taken to read affected “weed” lists, as sedges are considered weeds in crop systems. Always read and follow label instructions.
Pests: None noted.
Diseases: None noted.
- Seed production
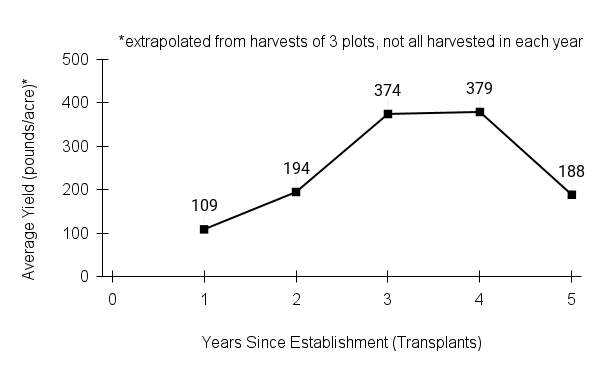 First harvest: First harvest is the year after transplanting.
First harvest: First harvest is the year after transplanting.Yield/acre: 100 to 380 pounds per acre (extrapolated from harvests of three plots)
Stand life: Stands remain productive for several years. We have seen a decline in production in some plots in their sixth year. Plots were retired from production after the sixth year, so it is uncertain whether or not they would have remained productive in the longer term.
Flowering date: June in northern Iowa
Seed maturity/Harvest date: late June to mid July in northern Iowa
Seed retention: Moderate risk of shattering. Ironically, the tendency of this species to lodge may protect somewhat against seed shatter.
Harvest date range at TPC (2008-2012): June 26 - July 18
Recommended harvest method: Combining is the most efficient method. However, lodging can be problematic, and we have hand harvested a badly lodged plot growing in wet-mesic soils after an exceptionally wet spring season.
- Seed cleaning and storage
Cleaning process: Air-dry seed for two weeks or more after harvest. For hand-harvested material, thresh seed from dried stalks by beating with rakes or forks, then treat similarly to combined material. Pass material through a coarse screen (1/2 in hardware cloth) to remove larger stemmy material, if needed, then air screen. If perigynia removal is desired, pass material through a brush machine with medium bristles before air screening. (Note: perigynia removal destroys several characteristics used in identification.)
Seed storage: cool/dry (33-50° F, 30-50% RH); seed may retain high viability for over 10 years under these conditions.
Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone 1 (northern Iowa) and Zone 2 (central Iowa).
- References
Boeck Crew, C.M., Myers, M.C., Sherrard, M.E., Elgersma, K.J., Houseal, G.A., & Smith D.D. (2020). Stratification and perigynia removal improve total germination and germination speed in 3 upland prairie sedge species. Native Plants Journal, 21(2), 120-131. https://doi.org/10.3368/npj.21.2.120
Chayka, K. (n.d.). Carex molesta (Troublesome Sedge). Minnesota Wildflowers. (n.d.). https://www.minnesotawildflowers.info/grass-sedge-rush/troublesome-sedge
Cochrane, T. S., Elliot, K., & Lipke, C. S. (2014). Field Oval Sedge. In Prairie Plants of the University of Wisconsin-Madison Arboretum (3rd ed., p. 163). essay, University of Wisconsin-Madison Arboretum.
Hilty, J. (2019). Troublesome Sedge. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/trouble_sedge.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
Mohlenbrock, R. H. (1999). Carex bicknellii. In Illustrated Flora of Illinois - Sedges: Carex (p. 139). Southern Illinois University Press.
Murphy, M. & Spyreas, G. & Marcum, P. (2025) Carex of Illinois & Surrounding States: The Oval Sedges. University of Illinois Press
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Smith, W.R. (2018) Sedges and Rushes of Minnesota: The Complete Guide to Species Identification. University of Minnesota Press.
USDA NRCS National Plant Data Team. (n.d.). Carex molesta Mack. ex Bright. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=CAMO11
Species Guide Updated 1/5/2026
woolgrass
woolgrass dickeye
Scirpus cyperinus, (L.) Kunth
Alternate Common Names: common woolsedge, woolgrass bulrush, cottongrass bulrush
Scientific Synonyms: Scirpus cyperinus (L.) Kunth var. condensatus Fernald, Scirpus cyperinus (L.) Kunth var. eriophorum (Michx.) Kunth, Scirpus cyperinus (L.) Kunth var. laxus (A. Gray) Beetle, Scirpus cyperinus (L.) Kunth var. pelius Fernald, Scirpus cyperinus (L.) Kunth var. rubricosus (Fernald) Gilly, Scirpus eriophorum Michx., Scirpus rubricosus Fernald, Scirpus cyperinus (L.) Kunth var. andrewsii (Fernald) Fernald, Eriophorum cyperinum L.
Family: sedge family (Cyperaceae)
Functional Group: sedges and rushes
Description
- Life cycle and growth form
Perennial with short rhizomes, forming dense, leafy clumps with tall flowering culms.
Height: 3-6 ft

- Leaves and stem
Leaves grass-like; flowering stems are smooth, round to subtly 3-sided, and sturdy; old leaves accumulate to form tussocks.
- Flower, fruit and seedhead
Flower/fruit/seed head: Clusters of 3-7 egg-shaped spikelets (each 1/3 in long) at the ends of slender branches in nodding, compound panicles 3-6 in long; spikelets are grayish green when in flower but mature to reddish brown; curly hairs on seeds expand when mature, making ripe seedheads look wooly.
Pollination: Wind

- Seed
Seed characteristics
Seed weight:
Seeds per ounce: 1,700,000 (IA NRCS)
1000 seed weight: 0.04g (Seed Information Database)
Description: “Seeds” are achenes that are 3-sided, oval-elliptical, pale gold, and tiny (less than 1 mm long and 0.5 mm wide). There is a tuft of wooly hairs at the base of each achene.
Typical seed test
PLS: 91%
Purity: 99%
Germination: 0%
Dormancy: 0%
(averages obtained from 1 tests of purchased seed lots)
- Habitat and range
Habitat: Moist to wet soil; partial to full sun; marshes, shorelines, swamps, seeps, sedge meadows, pond edges, ditches; Wetland Indicator Status is Obligate Wetland (OBL) for the Midwest; irrigation is needed for seed production.
Conservation status: Global- G5, secure; Nebraska- S1, critically imperiled; Kansas and Wyoming- S2, imperiled; Idaho and Montana- S3, vulnerable; in all other states within its natural range, status is S4 (apparently secure) to S5 (secure) or unranked (NatureServe).
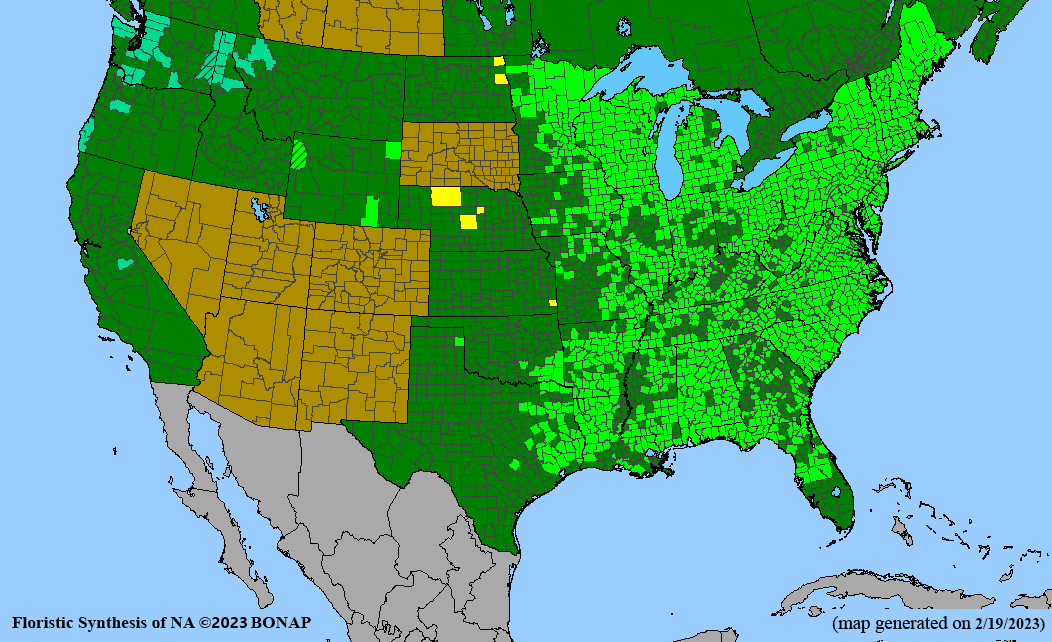
General Comments
The combination of wooly seedheads and clustered spikelets help distinguish woolgrass from similar bulrushes in the genus Scirpus. The robust, tussocky clumps of woolgrass protect soil from erosion in the wetlands and ditches where it thrives. Its leaves, stems, and seedheads provide food and nesting sites for waterfowl and other wildlife. This species is not difficult to propagate from seed, and grows well under irrigation, but the wooly hairs around its “seeds” (achenes) present some challenges for seed harvest and cleaning.
Recommendations for Seed Production
- Establishment for seed production
Direct seeding
Not recommended for this species.
Greenhouse
Seed pre-treatment: Cold/moist stratification for 30 days.
Sowing: About 3 months before the last frost, surface sow in germination flats (our preferred method) or directly into plugs. Aim for 2-3 seeds per cell, but this can be difficult to judge with such tiny seeds. In either type of container, use caution when watering to avoid splashing seed from the soil surface. Seedlings are extremely tiny but fast growing. If started in germination flats, they can be dibbled into plugs using forceps.
Transplanting: When seedlings have grown into well-rooted plugs, move them outside to harden off, then transplant into irrigated, plasticulture rows at 12 inch spacing.
- Stand management
Weeds: Prepare clean, weed-free beds and use plastic mulch to suppress weeds in the establishment year. Plants are robust and leafy, suppressing many weeds. Mow or cultivate between rows. Hand rogue to remove problematic weeds.
Pests: None noted.
Diseases: None noted.
- Seed production
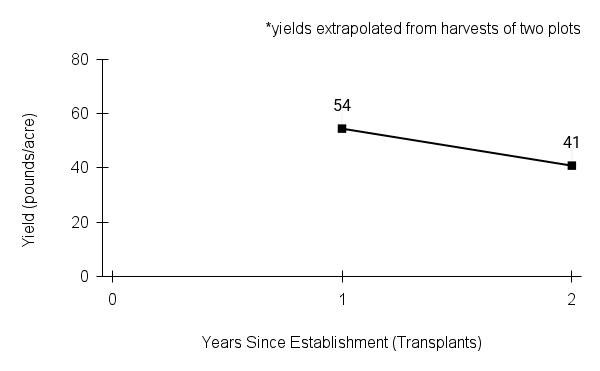 First harvest: Plants flower and set seed the year after planting.
First harvest: Plants flower and set seed the year after planting.Yield/acre: 40-60 pounds per acre (extrapolated from harvests of two production plots at TPC)
Stand life: Plants are likely long-lived, but we have not yet maintained plots for longer than three years. Yield from one plot declined from year 2 to year 3, and the plot was retired.
Flowering date: June in northern Iowa
Seed maturity/Harvest date: mid to late August in northern Iowa (the first harvest may occur later than normal)
Seed retention: Moderate risk of shattering when seedheads become “wooly.”
Harvest date range at TPC (2011-2025): August 14 - August 25
Recommended harvest method: Hand pick early maturing plants, then combine. We have had issues with the wooly hairs causing clumps to form on the combine sieves and pass out with the straw. We collected that material and passed it through a stationary combine to extract more seed.
- Seed cleaning and storage
Cleaning process: Brush with soft bristles to help break the wooly bristles from the achenes, then airscreen.
Seed storage: cool/dry (33-50° F, 30-50% RH)

Released Germplasm
Source Identified material: Natural Selections/Iowa Ecotype Zone Iowa
- References
Chayka, K. (n.d.). Scirpus cyperinus (woolgrass). Minnesota Wildflowers. https://www.minnesotawildflowers.info/grass-sedge-rush/woolgrass
Hilty, J. (2019). Wool grass - Scirpus cyperinus. Illinois Wildflowers. https://www.illinoiswildflowers.info/grasses/plants/wool_grass.htm
Kartesz, J.T., The Biota of North America Program (BONAP). 2023. North American Plant Atlas. (http://bonap.net/napa). Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2023. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]
NatureServe. 2024. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. (Accessed: February 29, 2024).
Schultz, Jan; Beyer, Patty; Williams, Julie. 2001. Propagation protocol for production of Container (plug) Scirpus cyperinus (L.) Kunth plants USDA FS - Hiawatha National Forest Marquette, Michigan. In: Native Plant Network. URL: https://NativePlantNetwork.org (accessed 2026/01/05). US Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources.
USDA NRCS National Plant Data Team. (n.d.). Scirpus cyperinus (L.) Kunth. USDA plants database. https://plants.usda.gov/home/plantProfile?symbol=SCCY
Whittemore, A. T. & Shuyler, A. E. (2020, November 5). Scirpus cyperinus (Linnaeus) Kunth. Flora of North America. http://floranorthamerica.org/Scirpus_cyperinus
Species Guide Updated 1/5/2026
Appendices
Appendices sagemThe third section provides extensive tables summarizing seed production information for native plant species which have been grown for seed production at the Tallgrass Prairie Center. Included are establishment recommendations, seed germination protocols, harvesting and seed cleaning information, sample seed test results, and seed counts per unit weight.
This section is under construction and new material will be added as soon as it is complete.
Appendix A
Appendix A sagemAppendix A is under construction. Check back later to find the following information:
- Table 1A - Establishment recommendations for seed production
- Table 2A - Seed pre-treatments for germination
Appendix B
Appendix B sagemAppendix B is under construction. Check back later to find the following information:
- Table 1B - Harvest characteristics and recommendations
Appendix C
Appendix C sagemSeed Cleaning Settings
The following files represent compilations of settings that have enabled us to clean seed of dozens of native prairie and wetland species at the Tallgrass Prairie Center. We use a Westrup laboratory scale brush machine and air screen cleaners for most seed cleaning tasks, and settings are provided for those machines. The settings are intended to provide a starting point from which you can modify the procedures and settings to suit your equipment and the characteristics of particular seedlots. You can download each file as a printable document (PDF) or as an Excel spreadsheet.
Settings for Westrup Laboratory Air Screen Cleaner
Settings for Westrup Laboratory Brush Machine
Appendix D
Appendix D sagemAppendix D is under construction. Check back later to find the following information:
- Table 1D - Typical seed test results
- Table 2D - Seed weight
- Table 3D - Soil moisture indications
- Table 4D - Relative degree of difficulty for seed increase
Supplemental Information
Supplemental Information sagemThis last section includes reference material for native plant propagation, native and weed species identification, and research publications on seed germination. Also provided is an abbreviated list of purveyors of seed-related equipment and horticultural supplies.
This section is under construction and new material will be added as soon as it is complete.
References
References sagemThis section is under construction and new material will be added as soon as it is complete.
Equipment
Equipment sagemThis section is under construction and new material will be added as soon as it is complete.